Expanded Clinical Phenotype, Oncological Associations, and Immunopathologic Insights of Paraneoplastic Kelch-like Protein-11 Encephalitis
- PMID: 32744608
- PMCID: PMC7653501
- DOI: 10.1001/jamaneurol.2020.2231
Expanded Clinical Phenotype, Oncological Associations, and Immunopathologic Insights of Paraneoplastic Kelch-like Protein-11 Encephalitis
Erratum in
-
Errors in Figures and Reference List.JAMA Neurol. 2020 Nov 1;77(11):1453. doi: 10.1001/jamaneurol.2020.3470. JAMA Neurol. 2020. PMID: 32926076 Free PMC article. No abstract available.
Abstract
Importance: Recognizing the presenting and immunopathological features of Kelch-like protein-11 immunoglobulin G seropositive (KLHL11 IgG+) patients may aid in early diagnosis and management.
Objective: To describe expanding neurologic phenotype, cancer associations, outcomes, and immunopathologic features of KLHL11 encephalitis.
Design, setting, and participants: This retrospective tertiary care center study, conducted from October 15, 1998, to November 1, 2019, prospectively identified 31 KLHL11 IgG+ cases in the neuroimmunology laboratory. Eight were identified by retrospective testing of patients with rhomboencephalitis (confirmed by tissue-based-immunofluorescence and transfected-cell-based assays).
Main outcomes and measures: Outcome variables included modified Rankin score and gait aid use.
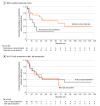
Results: All 39 KLHL11 IgG+ patients were men (median age, 46 years; range, 28-73 years). Initial clinical presentations were ataxia (n = 32; 82%), diplopia (n = 22; 56%), vertigo (n = 21; 54%), hearing loss (n = 15; 39%), tinnitus (n = 14; 36%), dysarthria (n = 11; 28%), and seizures (n = 9; 23%). Atypical neurologic presentations included neuropsychiatric dysfunction, myeloneuropathy, and cervical amyotrophy. Hearing loss or tinnitus preceded other neurologic deficits by 1 to 8 months in 10 patients (26%). Among patients screened for malignancy (n = 36), testicular germ-cell tumors (n = 23; 64%) or testicular microlithiasis and fibrosis concerning for regressed germ cell tumor (n = 7; 19%) were found in 83% of the patients (n = 30). In 2 patients, lymph node biopsy diagnosed metastatic lung adenocarcinoma in one and chronic lymphocytic leukemia in the other. Initial brain magnetic resonance imaging revealed T2 hyperintensities in the temporal lobe (n = 12), cerebellum (n = 9), brainstem (n = 3), or diencephalon (n = 3). Among KLHL11 IgG+ patients who underwent HLA class I and class II genotyping (n = 10), most were found to have HLA-DQB1*02:01 (n = 7; 70%) and HLA-DRB1*03:01 (n = 6; 60%) associations. A biopsied gadolinium-enhancing temporal lobe lesion demonstrated T cell-predominant inflammation and nonnecrotizing granulomas. Cerebellar biopsy (patient with chronic ataxia) and 2 autopsied brains demonstrated Purkinje neuronal loss and Bergmann gliosis, supporting early active inflammation and later extensive neuronal loss. Compared with nonautoimmune control peripheral blood mononuclear cells, cluster of differentiation (CD) 8+ and CD4+ T cells were significantly activated when patient peripheral blood mononuclear cells were cultured with KLHL11 protein. Most patients (58%) benefitted from immunotherapy and/or cancer treatment (neurological disability stabilized [n = 10] or improved [n = 9]). Kaplan-Meier curve demonstrated significantly higher probability of wheelchair dependence among patients without detectable testicular cancer. Long-term outcomes in KLHL11-IgG+ patients were similar to Ma2 encephalitis.
Conclusions and relevance: Kelch-like protein-11 IgG is a biomarker of testicular germ-cell tumor and paraneoplastic neurologic syndrome, often refractory to treatment. Described expanded neurologic phenotype and paraclinical findings may aid in its early diagnosis and treatment.
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

