Aromatic Linkers Unleash the Antiproliferative Potential of 3-Chloropiperidines Against Pancreatic Cancer Cells
- PMID: 32744774
- PMCID: PMC7692949
- DOI: 10.1002/cmdc.202000457
Aromatic Linkers Unleash the Antiproliferative Potential of 3-Chloropiperidines Against Pancreatic Cancer Cells
Abstract
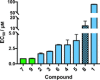
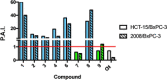
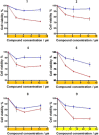
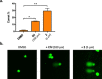
In this study, we describe the synthesis and biological evaluation of a set of bis-3-chloropiperidines (B-CePs) containing rigid aromatic linker structures. A modification of the synthetic strategy also enabled the synthesis of a pilot tris-3-chloropiperidine (Tri-CeP) bearing three reactive meta-chloropiperidine moieties on the aromatic scaffold. A structure-reactivity relationship analysis of B-CePs suggests that the arrangement of the reactive units affects the DNA alkylating activity, while also revealing correlations between the electron density of the aromatic system and the reactivity with biologically relevant nucleophiles, both on isolated DNA and in cancer cells. Interestingly, all aromatic 3-chloropiperidines exhibited a marked cytotoxicity and tropism for 2D and 3D cultures of pancreatic cancer cells. Therefore, the new aromatic 3-chloropiperidines appear to be promising contenders for further development of mustard-based anticancer agents aimed at pancreatic cancers.
Keywords: DNA damage; alkylating agents; aromatic chloropiperidines; pancreatic cancer spheroids; structure-activity relationships.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

