A gene expression-based single sample predictor of lung adenocarcinoma molecular subtype and prognosis
- PMID: 32745259
- PMCID: PMC7689824
- DOI: 10.1002/ijc.33242
A gene expression-based single sample predictor of lung adenocarcinoma molecular subtype and prognosis
Abstract
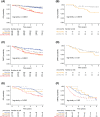
Disease recurrence in surgically treated lung adenocarcinoma (AC) remains high. New approaches for risk stratification beyond tumor stage are needed. Gene expression-based AC subtypes such as the Cancer Genome Atlas Network (TCGA) terminal-respiratory unit (TRU), proximal-inflammatory (PI) and proximal-proliferative (PP) subtypes have been associated with prognosis, but show methodological limitations for robust clinical use. We aimed to derive a platform independent single sample predictor (SSP) for molecular subtype assignment and risk stratification that could function in a clinical setting. Two-class (TRU/nonTRU=SSP2) and three-class (TRU/PP/PI=SSP3) SSPs using the AIMS algorithm were trained in 1655 ACs (n = 9659 genes) from public repositories vs TCGA centroid subtypes. Validation and survival analysis were performed in 977 patients using overall survival (OS) and distant metastasis-free survival (DMFS) as endpoints. In the validation cohort, SSP2 and SSP3 showed accuracies of 0.85 and 0.81, respectively. SSPs captured relevant biology previously associated with the TCGA subtypes and were associated with prognosis. In survival analysis, OS and DMFS for cases discordantly classified between TCGA and SSP2 favored the SSP2 classification. In resected Stage I patients, SSP2 identified TRU-cases with better OS (hazard ratio [HR] = 0.30; 95% confidence interval [CI] = 0.18-0.49) and DMFS (TRU HR = 0.52; 95% CI = 0.33-0.83) independent of age, Stage IA/IB and gender. SSP2 was transformed into a NanoString nCounter assay and tested in 44 Stage I patients using RNA from formalin-fixed tissue, providing prognostic stratification (relapse-free interval, HR = 3.2; 95% CI = 1.2-8.8). In conclusion, gene expression-based SSPs can provide molecular subtype and independent prognostic information in early-stage lung ACs. SSPs may overcome critical limitations in the applicability of gene signatures in lung cancer.
Keywords: gene expression; lung adenocarcinoma; molecular subtypes; prognosis; single sample predictor.
© 2020 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of Union for International Cancer Control.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures



References
-
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243‐1260. - PubMed
-
- Swanton C, Govindan R. Clinical implications of genomic discoveries in lung cancer. N Engl J Med. 2016;374:1864‐1873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

