Autophagy, the innate immune response and cancer
- PMID: 32745353
- PMCID: PMC7463325
- DOI: 10.1002/1878-0261.12774
Autophagy, the innate immune response and cancer
Abstract
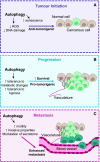
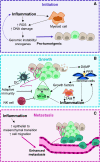
Autophagy is a cellular degradation and recycling system, which can interact with components of innate immune signalling pathways to enhance pathogen clearance, in both immune and nonimmune cells. Whilst this interaction is often beneficial for pathogen clearance, it can have varying outcomes in regard to tumorigenesis. Autophagy and the innate immune response can have both pro- and antitumorigenic effects at different stages of tumorigenesis due to the plastic nature of the tumour microenvironment (TME). Although both of these components have been studied in isolation as potential therapeutic targets, there has been less research concerning the interaction between autophagy and the innate immune response within the TME. As the innate immune response is critical for the formation of an effective antitumour adaptive immune response, targeting autophagy pathways in both tumour cells and innate immune cells could enhance tumour clearance. Within tumour cells, autophagy pathways are intertwined with pattern recognition receptor (PRR), inflammatory and cell death pathways, and therefore can alter the immunogenicity of the TME and development of the antitumour immune response. In innate immune cells, autophagy components can have autophagy-independent roles in functional pathways, and therefore could be valuable targets for enhancing immune cell function in the TME and immunotherapy. This review highlights the individual importance of autophagy and the innate immune response to tumorigenesis, and also explains the complex interactions between these pathways in the TME.
Keywords: autophagy; cancer; immunotherapy; innate immune response; tumour microenvironment.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no financial conflicts of interest to declare. KMR is Co‐Editor‐in‐Chief of
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

