Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs
- PMID: 32745595
- PMCID: PMC7834107
- DOI: 10.1016/j.cmi.2020.07.038
Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs
Abstract
Objectives: The aim was to determine the antibody response against SARS-CoV-2 spike protein and nucleoprotein using four automated immunoassays and three ELISAs for the detection of total Ig antibodies (Roche) or IgG (Abbott, Diasorin, Snibe, Euroimmun, Mikrogen) in COVID-19 patients.
Methods: Sensitivity and dynamic trend to seropositivity were evaluated in 233 samples from 114 patients with moderate, severe or critical COVID-19 confirmed with PCR on nasopharyngeal swab. Specificity was evaluated in 113 samples collected before January 2020, including 24 samples from patients with non-SARS coronavirus infection.
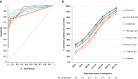
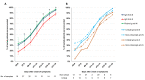
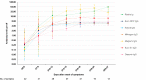
Results: Sensitivity for all assays was 100% (95% confidence interval 83.7-100) 3 weeks after onset of symptoms. Specificity varied between 94.7% (88.7-97.8) and 100% (96.1-100). Calculated at the cut-offs that corresponded to a specificity of 95% and 97.5%, Roche had the highest sensitivity (85.0% (79.8-89.0) and 81.1% (76.6-85.7), p < 0.05 except vs. Abbott). Seroconversion occurred on average 2 days earlier for Roche total Ig anti-N and the three IgG anti-N assays (Abbott, Mikrogen, Euroimmun) than for the two IgG anti-S assays (Diasorin, Euroimmun) (≥50% seroconversion day 9-10 vs. day 11-12 and p < 0.05 for percent seropositive patients day 9-10 to 17-18). There was no significant difference in the IgG antibody time to seroconversion between critical and non-critical patients.
Discussion: Seroconversion occurred within 3 weeks after onset of symptoms with all assays and on average 2 days earlier for assays detecting IgG or total Ig anti-N than for IgG anti-S. The specificity of assays detecting anti-N was comparable to anti-S and excellent in a challenging control population.
Keywords: COVID-19; Coronavirus; Diagnosis; ELISA; Immunoassay; Nucleocapsid protein; SARS-CoV-2; Sensitivity and specificity; Seroconversion; Spike glycoprotein.
Copyright © 2020 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L., Rattigan S.M., et al. A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. MedRxiv. 2020 doi: 10.1101/2020.04.14.20065771. (Epub ahead of print) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

