Stereotactic Transcranial Focused Ultrasound Targeting System for Murine Brain Models
- PMID: 32746229
- PMCID: PMC7814337
- DOI: 10.1109/TUFFC.2020.3012303
Stereotactic Transcranial Focused Ultrasound Targeting System for Murine Brain Models
Abstract
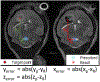
An inexpensive, accurate focused ultrasound stereotactic targeting method guided by pretreatment magnetic resonance imaging (MRI) images for murine brain models is presented. An uncertainty of each sub-component of the stereotactic system was analyzed. The entire system was calibrated using clot phantoms. The targeting accuracy of the system was demonstrated with an in vivo mouse glioblastoma (GBM) model. The accuracy was quantified by the absolute distance difference between the prescribed and ablated points visible on the pre treatment and posttreatment MR images, respectively. A precalibration phantom study ( N = 6 ) resulted in an error of 0.32 ± 0.31, 0.72 ± 0.16, and 1.06 ± 0.38 mm in axial, lateral, and elevational axes, respectively. A postcalibration phantom study ( N = 8 ) demonstrated a residual error of 0.09 ± 0.01, 0.15 ± 0.09, and 0.47 ± 0.18 mm in axial, lateral, and elevational axes, respectively. The calibrated system showed significantly reduced ( ) error of 0.20 ± 0.21, 0.34 ± 0.24, and 0.28 ± 0.21 mm in axial, lateral, and elevational axes, respectively, in the in vivo GBM tumor-bearing mice ( N = 10 ).
Figures





References
-
- Carter M and Shieh J, “Stereotaxic Surgeries and In Vivo Techniques,” Guid. to Res. Tech. Neurosci, no. Chapter 4, pp. 73–88, 2015.
-
- Willems PWA, Van Der Sprenkel JWB, Tulleken CAF, Viergever MA, and Taphoorn MJB, “Neuronavigation and surgery of intracerebral tumours,” J. Neurol, vol. 253, no. 9, pp. 1123–1136, 2006. - PubMed
-
- Omay SB and Barnett GH, “Surgical navigation for meningioma surgery,” J. Neurooncol, vol. 99, no. 3, pp. 357–364, 2010. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

