Clonal hematopoiesis: mechanisms driving dominance of stem cell clones
- PMID: 32746453
- PMCID: PMC7530644
- DOI: 10.1182/blood.2020006510
Clonal hematopoiesis: mechanisms driving dominance of stem cell clones
Abstract
The discovery of clonal hematopoiesis (CH) in older individuals has changed the way hematologists and stem cell biologists view aging. Somatic mutations accumulate in stem cells over time. While most mutations have no impact, some result in subtle functional differences that ultimately manifest in distinct stem cell behaviors. With a large pool of stem cells and many decades to compete, some of these differences confer advantages under specific contexts. Approximately 20 genes are recurrently found as mutated in CH, indicating they confer some advantage. The impact of these mutations has begun to be analyzed at a molecular level by modeling in cell lines and in mice. Mutations in epigenetic regulators such as DNMT3A and TET2 confer an advantage by enhancing self-renewal of stem and progenitor cells and inhibiting their differentiation. Mutations in other genes involved in the DNA damage response may simply enhance cell survival. Here, we review proposed mechanisms that lead to CH, specifically in the context of stem cell biology, based on our current understanding of the function of some of the CH-associated genes.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflicts-of-interest disclosure: M.A.G. and G.A.C. declare no competing financial interests.
Figures


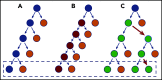

References
-
- Watson CJ, Papula AL, Poon GYP, et al. . The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science. 2020;367(6485):1449-1454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

