Postdischarge venous thromboembolism following hospital admission with COVID-19
- PMID: 32746455
- PMCID: PMC7483432
- DOI: 10.1182/blood.2020008086
Postdischarge venous thromboembolism following hospital admission with COVID-19
Abstract
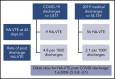
The association of severe coronavirus disease 2019 (COVID-19) with an increased risk of venous thromboembolism (VTE) has resulted in specific guidelines for its prevention and management. The VTE risk appears highest in those with critical care admission. The need for postdischarge thromboprophylaxis remains controversial, which is reflected in conflicting expert guideline recommendations. Our local protocol provides thromboprophylaxis to COVID-19 patients during admission only. We report postdischarge VTE data from an ongoing quality improvement program incorporating root-cause analysis of hospital-associated VTE (HA-VTE). Following 1877 hospital discharges associated with COVID-19, 9 episodes of HA-VTE were diagnosed within 42 days, giving a postdischarge rate of 4.8 per 1000 discharges. Over 2019, following 18 159 discharges associated with a medical admission; there were 56 episodes of HA-VTE within 42 days (3.1 per 1000 discharges). The odds ratio for postdischarge HA-VTE associated with COVID-19 compared with 2019 was 1.6 (95% confidence interval, 0.77-3.1). COVID-19 hospitalization does not appear to increase the risk of postdischarge HA-VTE compared with hospitalization with other acute medical illness. Given that the risk-benefit ratio of postdischarge thromboprophylaxis remains uncertain, randomized controlled trials to evaluate the role of continuing thromboprophylaxis in COVID-19 patients following hospital discharge are required.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: L.N.R. has received speaker fees and a travel grant from Bayer, and an investigator-initiated research grant and a travel grant from Sanofi. J.C. has received a travel grant from Mitsubishi Pharma and honoraria from Bayer and Sanofi. C.R. has received unrestricted research grants from Baxter, SOBI, BioMarin. B.V. has received travel grants and event sponsorship from Boehringer-Ingelheim and Bayer. R.K.P. has received speaker fees from Bayer. E.G. has received honoraria from Bayer. R.A. reports grants from Bayer; personal fees from Bayer, Pfizer, Medtronic, and Sanofi; and nonfinancial support from Bayer, Pfizer, and Sanofi. The remaining authors declare no competing financial interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources