Serologic responses to SARS-CoV-2 infection among hospital staff with mild disease in eastern France
- PMID: 32747185
- PMCID: PMC7502660
- DOI: 10.1016/j.ebiom.2020.102915
Serologic responses to SARS-CoV-2 infection among hospital staff with mild disease in eastern France
Abstract
Background: The serologic response of individuals with mild forms of SARS-CoV-2 infection is poorly characterized.
Methods: Hospital staff who had recovered from mild forms of PCR-confirmed SARS-CoV-2 infection were tested for anti-SARS-CoV-2 antibodies using two assays: a rapid immunodiagnostic test (99.4% specificity) and the S-Flow assay (~99% specificity). The neutralizing activity of the sera was tested with a pseudovirus-based assay.
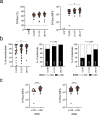
Findings: Of 162 hospital staff who participated in the investigation, 160 reported SARS-CoV-2 infection that had not required hospital admission and were included in these analyses. The median time from symptom onset to blood sample collection was 24 days (IQR: 21-28, range 13-39). The rapid immunodiagnostic test detected antibodies in 153 (95.6%) of the samples and the S-Flow assay in 159 (99.4%), failing to detect antibodies in one sample collected 18 days after symptom onset (the rapid test did not detect antibodies in that patient). Neutralizing antibodies (NAbs) were detected in 79%, 92% and 98% of samples collected 13-20, 21-27 and 28-41 days after symptom onset, respectively (P = 0.02).
Interpretation: Antibodies against SARS-CoV-2 were detected in virtually all hospital staff sampled from 13 days after the onset of COVID-19 symptoms. This finding supports the use of serologic testing for the diagnosis of individuals who have recovered from SARS-CoV-2 infection. The neutralizing activity of the antibodies increased overtime. Future studies will help assess the persistence of the humoral response and its associated neutralization capacity in recovered patients.
Fundings: The funders had no role in study design, data collection, interpretation, or the decision to submit the work for publication.
Keywords: Antibodies; Mild covid-19; Neutralization; Serology; sars-cov-2.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest SFK, YM, RG, LT, FA, PS, CSM, NC, AB, AV, NL, MM, NM, DR.., BH, JDS and AF have no competing interest to declare. PC is the founder and CSO of TheraVectys. LG, IS, TB, and OS are holder of a provisional patent on the S-Flow assay. Dr. Schwartz has a patent "Methods and products for serological analysis of SARS-COV-2 Infection" pending on the S-Flow assay. Dr. Rey reports grants and personal fees from Mylan, personal fees from ViiV Healthcare, grants from Gilead, grants from Abbvie, outside the submitted work.
Figures
Comment in
-
Antibody responses in mild COVID-19 hospital staff.EBioMedicine. 2020 Sep;59:102940. doi: 10.1016/j.ebiom.2020.102940. Epub 2020 Aug 15. EBioMedicine. 2020. PMID: 32807702 Free PMC article. No abstract available.
Similar articles
-
Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR.EBioMedicine. 2020 Sep;59:102960. doi: 10.1016/j.ebiom.2020.102960. Epub 2020 Aug 24. EBioMedicine. 2020. PMID: 32853988 Free PMC article.
-
COVID-19 and lombardy: TESTing the impact of the first wave of the pandemic.EBioMedicine. 2020 Nov;61:103069. doi: 10.1016/j.ebiom.2020.103069. Epub 2020 Oct 22. EBioMedicine. 2020. PMID: 33130396 Free PMC article.
-
Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study.Lancet Infect Dis. 2020 May;20(5):565-574. doi: 10.1016/S1473-3099(20)30196-1. Epub 2020 Mar 23. Lancet Infect Dis. 2020. PMID: 32213337 Free PMC article.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
-
Types of Assays for SARS-CoV-2 Testing: A Review.Lab Med. 2020 Sep 1;51(5):e59-e65. doi: 10.1093/labmed/lmaa039. Lab Med. 2020. PMID: 32657343 Free PMC article. Review.
Cited by
-
What Is the Antibody Response and Role in Conferring Natural Immunity After SARS-CoV-2 Infection? Rapid, Living Practice Points From the American College of Physicians (Version 1).Ann Intern Med. 2021 Jun;174(6):828-835. doi: 10.7326/M20-7569. Epub 2021 Mar 16. Ann Intern Med. 2021. Update in: Ann Intern Med. 2022 Apr;175(4):556-565. doi: 10.7326/M21-3272. PMID: 33721518 Free PMC article. Updated.
-
A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations.Sci Transl Med. 2020 Sep 2;12(559):eabc3103. doi: 10.1126/scitranslmed.abc3103. Epub 2020 Aug 17. Sci Transl Med. 2020. PMID: 32817357 Free PMC article.
-
Real-time seroprevalence and exposure levels of emerging pathogens in infection-naive host populations.Sci Rep. 2021 Mar 12;11(1):5825. doi: 10.1038/s41598-021-84672-1. Sci Rep. 2021. PMID: 33712648 Free PMC article.
-
How should a positive PCR test result for COVID-19 in an asymptomatic individual be interpreted and managed?Med Mal Infect. 2020 Nov;50(8):633-638. doi: 10.1016/j.medmal.2020.09.014. Epub 2020 Oct 3. Med Mal Infect. 2020. PMID: 33022291 Free PMC article. Review. No abstract available.
-
Robust and Persistent B- and T-Cell Responses after COVID-19 in Immunocompetent and Solid Organ Transplant Recipient Patients.Viruses. 2021 Nov 11;13(11):2261. doi: 10.3390/v13112261. Viruses. 2021. PMID: 34835067 Free PMC article.
References
-
- To K.K.-.W., Tsang O.T.-.Y., Leung W.-.S., Tam A.R., Wu T.-.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/s1473-3099(20)30196-1. - DOI - PMC - PubMed
-
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature2020:1–5. doi:10.1038/s41586-020-2196-x. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

