Pathogenesis of COVID-19-induced ARDS: implications for an ageing population
- PMID: 32747391
- PMCID: PMC7397945
- DOI: 10.1183/13993003.02049-2020
Pathogenesis of COVID-19-induced ARDS: implications for an ageing population
Abstract
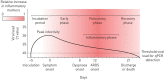
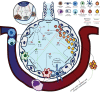
The coronavirus disease 2019 (COVID-19) pandemic has elicited a swift response by the scientific community to elucidate the pathogenesis of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2)-induced lung injury and develop effective therapeutics. Clinical data indicate that severe COVID-19 most commonly manifests as viral pneumonia-induced acute respiratory distress syndrome (ARDS), a clinical entity mechanistically understood best in the context of influenza A virus-induced pneumonia. Similar to influenza, advanced age has emerged as the leading host risk factor for developing severe COVID-19. In this review we connect the current understanding of the SARS-CoV-2 replication cycle and host response to the clinical presentation of COVID-19, borrowing concepts from influenza A virus-induced ARDS pathogenesis and discussing how these ideas inform our evolving understanding of COVID-19-induced ARDS. We also consider important differences between COVID-19 and influenza, mainly the protean clinical presentation and associated lymphopenia of COVID-19, the contrasting role of interferon-γ in mediating the host immune response to these viruses, and the tropism for vascular endothelial cells of SARS-CoV-2, commenting on the potential limitations of influenza as a model for COVID-19. Finally, we explore hallmarks of ageing that could explain the association between advanced age and susceptibility to severe COVID-19.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: M.A. Torres Acosta has nothing to disclose. Conflict of interest: B.D. Singer has a patent US Patent App. 15/542,380, “Compositions and Methods to Accelerate Resolution of Acute Lung Inflammation” pending.
Figures
References
-
- World Health Organization . Coronavirus disease (COVID-19) pandemic. www.who.int/emergencies/diseases/novel-coronavirus-2019 Date last accessed: 10 July 2020.
-
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020; 323: 1775–1776 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
