Development and Validation of the Elecsys Anti-SARS-CoV-2 Immunoassay as a Highly Specific Tool for Determining Past Exposure to SARS-CoV-2
- PMID: 32747400
- PMCID: PMC7512151
- DOI: 10.1128/JCM.01694-20
Development and Validation of the Elecsys Anti-SARS-CoV-2 Immunoassay as a Highly Specific Tool for Determining Past Exposure to SARS-CoV-2
Abstract
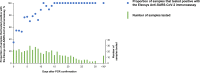
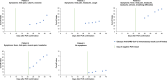
The Elecsys Anti-SARS-CoV-2 immunoassay (Roche Diagnostics) was developed to provide accurate, reliable detection of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We evaluated sensitivity, specificity, cross-reactivity, and agreement with a vesicular stomatitis virus-based pseudoneutralization assay for the Elecsys Anti-SARS-CoV-2 immunoassay. Sensitivity and agreement between Elecsys Anti-SARS-CoV-2 immunoassay and pseudoneutralization assay measurements were evaluated using samples from patients with PCR-confirmed SARS-CoV-2 infection, a majority of whom were hospitalized. Specificity was evaluated using samples from routine diagnostic testing/blood donors collected before December 2019 and thus deemed negative for SARS-CoV-2-specific antibodies. Cross-reactivity was evaluated using samples containing a wide range of potentially cross-reacting analytes, purchased from commercial vendors. For sensitivity and specificity, point estimates and 95% confidence intervals (CIs) were calculated. Agreement between the Elecsys Anti-SARS-CoV-2 immunoassay and the pseudoneutralization assay was calculated. The sensitivity of the Elecsys Anti-SARS-CoV-2 immunoassay in patients with prior PCR-confirmed SARS-CoV-2 infection was 99.5% (95% CI, 97.0 to 100.0%) at ≥14 days post-PCR confirmation. Overall specificity (n = 10,453) was 99.80% (95% CI, 99.69 to 99.88%). Only 4/792 samples containing potential cross-reacting analytes were reactive with the Elecsys Anti-SARS-CoV-2 immunoassay, resulting in an overall specificity in this cohort of 99.5% (95% CI, 98.6 to 99.9%). Positive, negative, and overall agreement (n = 46) between the Elecsys Anti-SARS-CoV-2 immunoassay and the pseudoneutralization assay were 86.4% (95% CI, 73.3 to 93.6%), 100% (95% CI, 34.2 to 100%), and 87.0% (95% CI, 74.3 to 93.9%), respectively. The Elecsys Anti-SARS-CoV-2 immunoassay demonstrated high sensitivity (99.5% at ≥14 days post-PCR confirmation) and specificity (99.80%), supporting its use as a tool for identification of past SARS-CoV-2 infection, including use in populations with low disease prevalence.
Keywords: SARS-CoV-2; cross-reactivity; diagnosis; immunoassay; neutralization testing; sensitivity; specificity.
Copyright © 2020 Muench et al.
Figures
References
-
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207. doi: 10.1056/NEJMoa2001316. - DOI - PMC - PubMed
-
- World Health Organization. 2020. Pneumonia of unknown cause—China. Disease outbreak news, 5 January 2020. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-ch....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

