Green Apple e-Cigarette Flavorant Farnesene Triggers Reward-Related Behavior by Promoting High-Sensitivity nAChRs in the Ventral Tegmental Area
- PMID: 32747456
- PMCID: PMC7433896
- DOI: 10.1523/ENEURO.0172-20.2020
Green Apple e-Cigarette Flavorant Farnesene Triggers Reward-Related Behavior by Promoting High-Sensitivity nAChRs in the Ventral Tegmental Area
Abstract
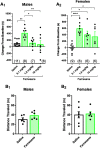
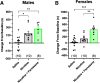
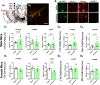
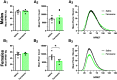
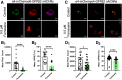
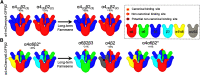
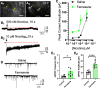
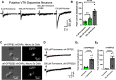
While combustible cigarette smoking has declined, the use of electronic nicotine delivery systems (ENDS) has increased. ENDS are popular among adolescents, and chemical flavorants are an increasing concern because of the growing use of zero-nicotine flavored e-liquids. Despite this, little is known regarding the effects of ENDS flavorants on vaping-related behavior. Following previous studies demonstrating the green apple flavorant, farnesol, enhances nicotine reward and exhibits rewarding properties without nicotine, this work focuses on the green apple flavorant, farnesene, for its impact on vaping-related behaviors. Using adult C57BL/6J mice, genetically modified to contain fluorescent nicotinic acetylcholine receptors (nAChRs), and farnesene doses of 0.1, 1.0, and 10 mg/kg, we observed farnesene-alone produces reward-related behavior in both male and female mice. We then performed whole-cell patch-clamp electrophysiology and observed farnesene-induced inward currents in ventral tegmental area (VTA) putative dopamine (pDA) neurons that were blocked by the nAChR antagonist, DhβE. While the amplitudes of farnesene-induced currents are ∼30% of nicotine's efficacy, this indicates the potential for some ENDS flavorants to stimulate nAChR function. Additionally, farnesene enhances nicotine's potency for activating nAChRs on VTA dopamine neurons. This may be because of changes in nAChR stoichiometry as our data suggest a shift toward high-sensitivity α4β2 nAChRs. Consequently, these data show that the green apple flavorant, farnesene, causes reward-related behavior without nicotine through changes in nAChR stoichiometry that results in an enhanced effect of nicotine on VTA dopamine neurons. These results demonstrate the importance of future investigations into ENDS flavorants and their effects on vaping-related behaviors.
Keywords: electrophysiology; flavorants; microscopy; nicotinic receptors; reward-related behavior.
Copyright © 2020 Cooper et al.
Figures
References
-
- Avelar AJ, Akers AT, Baumgard ZJ, Cooper SY, Casinelli GP, Henderson BJ (2019) Why flavored vape products may be attractive: green apple tobacco flavor elicits reward-related behavior, upregulates nAChRs on VTA dopamine neurons, and alters midbrain dopamine and GABA neuron function. Neuropharmacology 158:107729 10.1016/j.neuropharm.2019.107729 - DOI - PMC - PubMed
-
- Brody A, Mukhin A, La Charite J, Ta K, Farahi J, Sugar C, Mamoun M, Vellios E, Archie M, Kozman M, Phuong J, Arlorio F, Mandelkern M (2013) Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol 16:957–966. 10.1017/S1461145712001022 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
