Follicular helper T cell profiles predict response to costimulation blockade in type 1 diabetes
- PMID: 32747817
- PMCID: PMC7610476
- DOI: 10.1038/s41590-020-0744-z
Follicular helper T cell profiles predict response to costimulation blockade in type 1 diabetes
Abstract
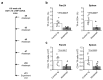
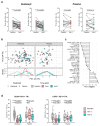
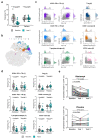
Follicular helper T (TFH) cells are implicated in type 1 diabetes (T1D), and their development has been linked to CD28 costimulation. We tested whether TFH cells were decreased by costimulation blockade using the CTLA-4-immunoglobulin (Ig) fusion protein (abatacept) in a mouse model of diabetes and in individuals with new-onset T1D. Unbiased bioinformatics analysis identified that inducible costimulatory molecule (ICOS)+ TFH cells and other ICOS+ populations, including peripheral helper T cells, were highly sensitive to costimulation blockade. We used pretreatment TFH profiles to derive a model that could predict clinical response to abatacept in individuals with T1D. Using two independent approaches, we demonstrated that higher frequencies of ICOS+ TFH cells at baseline were associated with a poor clinical response following abatacept administration. Therefore, TFH analysis may represent a new stratification tool, permitting the identification of individuals most likely to benefit from costimulation blockade.
Conflict of interest statement
AstraZeneca plc contributed to the funding of the study. P.A. and L.J. declare an interest in developing costimulation blockade reagents at AstraZeneca plc. L.S.K.W. and N.T. are inventors on a patent application related to these findings.
Figures





Comment in
-
Circulating TFH cells as a marker for early therapeutic intervention in T1D.Nat Immunol. 2020 Oct;21(10):1141-1142. doi: 10.1038/s41590-020-0766-6. Nat Immunol. 2020. PMID: 32873972 No abstract available.
-
A machine learning approach to predict response to immunotherapy in type 1 diabetes.Cell Mol Immunol. 2021 Mar;18(3):515-517. doi: 10.1038/s41423-020-00594-4. Epub 2020 Dec 14. Cell Mol Immunol. 2021. PMID: 33318626 Free PMC article. No abstract available.
References
-
- Blair HA, Deeks ED. Abatacept: A Review in Rheumatoid Arthritis. Drugs. 2017;77:1221–1233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK061010/DK/NIDDK NIH HHS/United States
- U01 DK061040/DK/NIDDK NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- U01 DK085463/DK/NIDDK NIH HHS/United States
- U01 DK061055/DK/NIDDK NIH HHS/United States
- U01 DK061036/DK/NIDDK NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- U01 DK061041/DK/NIDDK NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- U01 DK085505/DK/NIDDK NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- 15/0005253/DUK_/Diabetes UK/United Kingdom
- MR/N001435/1/MRC_/Medical Research Council/United Kingdom
- MR/S009140/1/MRC_/Medical Research Council/United Kingdom
- U01 DK061034/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

