Clofazimine pharmacokinetics in patients with TB: dosing implications
- PMID: 32747933
- PMCID: PMC7566350
- DOI: 10.1093/jac/dkaa310
Clofazimine pharmacokinetics in patients with TB: dosing implications
Abstract
Background: Clofazimine is in widespread use as a key component of drug-resistant TB regimens, but the recommended dose is not evidence based. Pharmacokinetic data from relevant patient populations are needed to inform dose optimization.
Objectives: To determine clofazimine exposure, evaluate covariate effects on variability, and simulate exposures for different dosing strategies in South African TB patients.
Patients and methods: Clinical and pharmacokinetic data were obtained from participants with pulmonary TB enrolled in two studies with intensive and sparse sampling for up to 6 months. Plasma concentrations were measured by LC-MS/MS and interpreted with non-linear mixed-effects modelling. Body size descriptors and other potential covariates were tested on pharmacokinetic parameters. We simulated different dosing regimens to safely shorten time to average daily concentration above a putative target concentration of 0.25 mg/L.
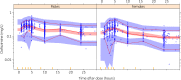
Results: We analysed 1570 clofazimine concentrations from 139 participants; 79 (57%) had drug-resistant TB and 54 (39%) were HIV infected. Clofazimine pharmacokinetics were well characterized by a three-compartment model. Clearance was 11.5 L/h and peripheral volume 10 500 L for a typical participant. Lower plasma exposures were observed in women during the first few months of treatment, explained by higher body fat fraction. Model-based simulations estimated that a loading dose of 200 mg daily for 2 weeks would achieve average daily concentrations above a target efficacy concentration 37 days earlier in a typical TB participant.
Conclusions: Clofazimine was widely distributed with a long elimination half-life. Disposition was strongly influenced by body fat content, with potential dosing implications for women with TB.
© The Author(s) 2020. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures




References
-
- WHO. Global Tuberculosis Report 2019 https://www.who.int/tb/publications/global_report/en/.
-
- WHO. Rapid Communication: Key Changes to Treatment of Drug-Resistant Tuberculosis https://www.who.int/tb/publications/2019/rapid_communications_MDR/en/.
-
- WHO. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-re.... - PubMed
-
- O’Donnell MR, Padayatchi N, Metcalfe JZ.. Elucidating the role of clofazimine for the treatment of tuberculosis. Int J Tuberc Lung Dis 2016; 20: 52–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 098316/WT_/Wellcome Trust/United Kingdom
- U19 AI111211/AI/NIAID NIH HHS/United States
- 203135/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- 203135/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- 098316 /WT_/Wellcome Trust/United Kingdom
- UL1 TR001073/TR/NCATS NIH HHS/United States
- R01 AI114304/AI/NIAID NIH HHS/United States
- K43 TW011421/TW/FIC NIH HHS/United States
- P30 AI124414/AI/NIAID NIH HHS/United States
- UL1 TR002556/TR/NCATS NIH HHS/United States
- K24 AI155045/AI/NIAID NIH HHS/United States
- K24 AI114444/AI/NIAID NIH HHS/United States

