TAS-303 effects on urethral sphincter function in women with stress urinary incontinence: phase I study
- PMID: 32747975
- PMCID: PMC7902327
- DOI: 10.1007/s00192-020-04470-7
TAS-303 effects on urethral sphincter function in women with stress urinary incontinence: phase I study
Abstract
Introduction and hypothesis: TAS-303, which selectively inhibits noradrenaline reuptake, was developed for treating stress urinary incontinence (SUI). The proximal urethra mainly comprises smooth muscle fibers in which α1 adrenergic receptors are abundant. This study was conducted to evaluate the effect of TAS-303 on urethral function and its safety profile in female patients with SUI.
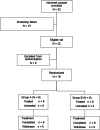
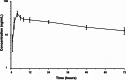
Methods: In total, 16 women (age, 20-64 years) with SUI and > 5.0 g of leakage in the 1-h pad test at screening were randomized and administered the assigned treatment in a double-blind manner. The primary end point was change in the maximal urethral closure pressure (MUCP) at 6 h post-dose. The secondary end point was change in the urethral closure pressure of the entire urethra and each urethral region (proximal, middle, and distal) at 6 h post-dose. The results were analyzed using a t-test.
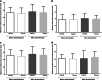
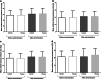
Results: The mean change ± standard deviation in MUCP at 6 h post-dose was 3.473 ± 12.154 cmH2O for TAS-303 and 2.615 ± 9.794 cmH2O for placebo (between-group difference: 0.858 cmH2O, P = 0.8047). The mean changes ± standard deviation in urethral closure pressure of the proximal urethra at 6 h after the administration of TAS-303 18 mg and placebo were 3.863 ± 10.941 and 1.634 ± 12.093, respectively (between-group difference: 2.229 cmH2O, P = 0.5976).
Conclusions: No significant difference in MUCP and urethral closure pressure was found between TAS-303 and placebo. However, the change in the proximal urethral closure pressure with TAS-303 was larger than that with placebo. This suggests that TAS-303 has pharmacological effects on urethral sphincteric function.
Keywords: Japan; Maximum urethral closure pressure; Randomized controlled study; Stress urinary incontinence; TAS-303; Urethral pressure profile parameters.
Conflict of interest statement
MG declares having received consultancy fees from Taiho, Astellas, Kowa, and Kyorin and grants from Taiho, Pfizer, Astellas, Daiichi Sankyo, Nihon Kayaku, Nippon Shinyaku, Takeda, and Asahikasei Pharma. All remaining authors have no conflicts of interest to declare.
Figures
References
-
- The International Continence Society (ICS) glossary of terminology. https://www.ics.org/glossary/symptom/stressurinaryincontinence. Accessed 24 Dec 2019.
-
- Fusco F, Abdel-Fattah M, Chapple CR, Creta M, La Falce S, Waltregny D, Novara G. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral Tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2017;72:567–591. doi: 10.1016/j.eururo.2017.04.026. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

