Sleep, brain vascular health and ageing
- PMID: 32748314
- PMCID: PMC7525637
- DOI: 10.1007/s11357-020-00235-8
Sleep, brain vascular health and ageing
Abstract
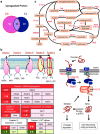
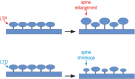
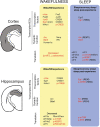
Sleep maintains the function of the entire body through homeostasis. Chronic sleep deprivation (CSD) is a prime health concern in the modern world. Previous reports have shown that CSD has profound negative effects on brain vasculature at both the cellular and molecular levels, and that this is a major cause of cognitive dysfunction and early vascular ageing. However, correlations among sleep deprivation (SD), brain vascular changes and ageing have barely been looked into. This review attempts to correlate the alterations in the levels of major neurotransmitters (acetylcholine, adrenaline, GABA and glutamate) and signalling molecules (Sirt1, PGC1α, FOXO, P66shc, PARP1) in SD and changes in brain vasculature, cognitive dysfunction and early ageing. It also aims to connect SD-induced loss in the number of dendritic spines and their effects on alterations in synaptic plasticity, cognitive disabilities and early vascular ageing based on data available in scientific literature. To the best of our knowledge, this is the first article providing a pathophysiological basis to link SD to brain vascular ageing.
Keywords: Cognition; Neurochemicals; Sleep deprivation; Synaptic plasticity; Vascular ageing.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

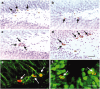
References
-
- Aggarwal B, Nour M, Riddhi S, Memet E, Ying W, Marie-Pierre S-O, Sanja J. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: findings from the American Heart Association go red for women strategically focused research network. J Am Heart Assoc. 2018;7:e008590. - PMC - PubMed
-
- Alborch E, Torregrosa G, Terrasa JC, Estrada C. GABA receptors mediate cerebral vasodilation in the unanesthetized goat. Brain Res. 1984;321:103–110. - PubMed
-
- Alexopoulou C, Kondili E, Diamantaki E, Psarologakis C, Kokkini S, Bolaki M, Georgopoulos D. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology. 2014;121:801–807. - PubMed
-
- Anafi RC, Kayser MS, Raizen DM. Exploring phylogeny to find the function of sleep. Nat Rev Neurosci. 2019;20:109. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

