Satellite cell self-renewal in endurance exercise is mediated by inhibition of mitochondrial oxygen consumption
- PMID: 32748470
- PMCID: PMC7749620
- DOI: 10.1002/jcsm.12601
Satellite cell self-renewal in endurance exercise is mediated by inhibition of mitochondrial oxygen consumption
Abstract
Background: Skeletal muscle stem cells (satellite cells) are well known to participate in regeneration and maintenance of the tissue over time. Studies have shown increases in the number of satellite cells after exercise, but their functional role in endurance training remains unexplored.
Methods: Young adult mice were submitted to endurance exercise training and the function, differentiation, and metabolic characteristics of satellite cells were investigated in vivo and in vitro.
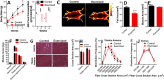
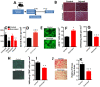
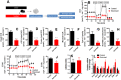
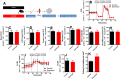
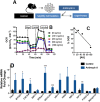
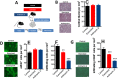
Results: We found that injured muscles from endurance-exercised mice display improved regenerative capacity, demonstrated through higher densities of newly formed myofibres compared with controls (evidenced by an increase in embryonic myosin heavy chain expression), as well as lower inflammation (evidenced by quantifying CD68-marked macrophages), and reduced fibrosis. Enhanced myogenic function was accompanied by an increased fraction of satellite cells expressing self-renewal markers, while control satellite cells had morphologies suggestive of early differentiation. The beneficial effects of endurance exercise were associated with satellite cell metabolic reprogramming, including reduced mitochondrial respiration (O2 consumption) under resting conditions (absence of muscle injury) and increased stemness. During proliferation or activated states (3 days after injury), O2 consumption was equal in control and exercised cells, while exercise enhanced myogenic colony formation. Surprisingly, inhibition of mitochondrial O2 consumption was sufficient to enhance muscle stem cell self-renewal characteristics in vitro. Moreover, transplanted muscle satellite cells from exercised mice or cells with reduced mitochondrial respiration promoted a significant reduction in inflammation compared with controls.
Conclusions: Our results indicate that endurance exercise promotes self-renewal and inhibits differentiation in satellite cells, an effect promoted by metabolic reprogramming and respiratory inhibition, which is associated with a more favourable muscular response to injury.
Keywords: Bioenergetics; Metabolism; Mitochondria; Muscle stem cells.
© 2020 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
None declared.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

