Tailored design of protein nanoparticle scaffolds for multivalent presentation of viral glycoprotein antigens
- PMID: 32748788
- PMCID: PMC7402677
- DOI: 10.7554/eLife.57659
Tailored design of protein nanoparticle scaffolds for multivalent presentation of viral glycoprotein antigens
Abstract
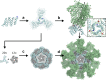
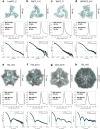
Multivalent presentation of viral glycoproteins can substantially increase the elicitation of antigen-specific antibodies. To enable a new generation of anti-viral vaccines, we designed self-assembling protein nanoparticles with geometries tailored to present the ectodomains of influenza, HIV, and RSV viral glycoprotein trimers. We first de novo designed trimers tailored for antigen fusion, featuring N-terminal helices positioned to match the C termini of the viral glycoproteins. Trimers that experimentally adopted their designed configurations were incorporated as components of tetrahedral, octahedral, and icosahedral nanoparticles, which were characterized by cryo-electron microscopy and assessed for their ability to present viral glycoproteins. Electron microscopy and antibody binding experiments demonstrated that the designed nanoparticles presented antigenically intact prefusion HIV-1 Env, influenza hemagglutinin, and RSV F trimers in the predicted geometries. This work demonstrates that antigen-displaying protein nanoparticles can be designed from scratch, and provides a systematic way to investigate the influence of antigen presentation geometry on the immune response to vaccination.
Keywords: B lymphocytes; BL21; E. coli; HEK293F; Lemo21; computational biology; human; immunology; inflammation; systems biology; virus.
Plain language summary
Vaccines train the immune system to recognize a specific virus or bacterium so that the body can be better prepared against these harmful agents. To do so, many vaccines contain viral molecules called glycoproteins, which are specific to each type of virus. Glycoproteins that sit at the surface of the virus can act as ‘keys’ that recognize and unlock the cells of certain organisms, leading to viral infection. To ensure a stronger immune response, glycoproteins in vaccines are often arranged on a protein scaffolding which can mimic the shape of the virus of interest and trigger a strong immune response. Many scaffoldings, however, are currently made from natural proteins which cannot always display viral glycoproteins. Here, Ueda, Antanasijevic et al. developed a method that allows for the design of artificial proteins which can serve as scaffolding for viral glycoproteins. This approach was tested using three viruses: influenza, HIV, and RSV – a virus responsible for bronchiolitis. The experiments showed that in each case, the relevant viral glycoproteins could attach themselves to the scaffolding. These structures could then assemble themselves into vaccine particles with predicted geometrical shapes, which mimicked the virus and maximized the response from the immune system. Designing artificial scaffolding for viral glycoproteins gives greater control over vaccine design, allowing scientists to manipulate the shape of vaccine particles and test the impact on the immune response. Ultimately, the approach developed by Ueda, Antanasijevic et al. could lead to vaccines that are more efficient and protective, including against viruses for which there is currently no suitable scaffolding.
Conflict of interest statement
GU, JF Inventor on U.S. patent application 62/422,872 titled “Computational design of self-assembling cyclic protein homo-oligomers.” Inventor on U.S. patent application 62/636,757 titled “Method of multivalent antigen presentation on designed protein nanomaterials.” Inventor on U.S. patent application PCT/US20/17216 titled “Nanoparticle-based Influenza Virus Vaccines and Uses Thereof.”, WS Inventor on U.S. patent application 62/422,872 titled “Computational design of self-assembling cyclic protein homo-oligomers.”, JC, GH, AM, AY, YT, YP, MB, BS, RG, PB, PZ, DV, RS, JM, PK, AW No competing interests declared, DE Inventor on U.S. patent application 62/636,757 titled “Method of multivalent antigen presentation on designed protein nanomaterials.” Inventor on U.S. patent application PCT/US20/17216 titled “Nanoparticle-based Influenza Virus Vaccines and Uses Thereof.”, MK Inventor on U.S. patent application PCT/US20/17216 titled “Nanoparticle-based Influenza Virus Vaccines and Uses Thereof.”, BG Inventor on U.S. patent application PCT/US20/17216 titled “Nanoparticle-based Influenza Virus Vaccines and Uses Thereof.” Member of Icosavax’s Scientific Advisory Board. NK Inventor on U.S. patent application 62/636,757 titled “Method of multivalent antigen presentation on designed protein nanomaterials.” Inventor on U.S. patent application PCT/US20/17216 titled “Nanoparticle-based Influenza Virus Vaccines and Uses Thereof.” Co-founder and shareholder of Icosavax, a company that has licensed these patent applications. Member of Icosavax’s Scientific Advisory Board. DB Inventor on U.S. patent application 62/422,872 titled “Computational design of self-assembling cyclic protein homo-oligomers.” Inventor on U.S. patent application 62/636,757 titled “Method of multivalent antigen presentation on designed protein nanomaterials.” Inventor on U.S. patent application PCT/US20/17216 titled “Nanoparticle-based Influenza Virus Vaccines and Uses Thereof.” Co-founder and shareholder of Icosavax, a company that has licensed these patent applications. Member of Icosavax’s Scientific Advisory Board.
Figures











References
-
- Abbott RK, Lee JH, Menis S, Skog P, Rossi M, Ota T, Kulp DW, Bhullar D, Kalyuzhniy O, Havenar-Daughton C, Schief WR, Nemazee D, Crotty S. Precursor frequency and affinity determine B cell competitive fitness in germinal centers, tested with Germline-Targeting HIV vaccine immunogens. Immunity. 2018;48:133–146. doi: 10.1016/j.immuni.2017.11.023. - DOI - PMC - PubMed
-
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallographica Section D Biological Crystallography. 2012;68:352–367. doi: 10.1107/S0907444912001308. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- OPP1156262/Bill and Melinda Gates Foundation/International
- P41 GM103403/GM/NIGMS NIH HHS/United States
- DE-AC02-05CH11231/U.S. Department of Energy/International
- S10 OD023476/OD/NIH HHS/United States
- CHE 1629214/National Science Foundation/International
- OPP1111923/Bill and Melinda Gates Foundation/International
- OPP1115782/Bill and Melinda Gates Foundation/International
- P01 AI110657/AI/NIAID NIH HHS/United States
- P30 GM124169/GM/NIGMS NIH HHS/United States
- P41 RR015301/RR/NCRR NIH HHS/United States
- OPP1120319/Bill and Melinda Gates Foundation/International
- S10 OD018483/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

