Constituents of Huberantha jenkinsii and Their Biological Activities
- PMID: 32748832
- PMCID: PMC7435746
- DOI: 10.3390/molecules25153533
Constituents of Huberantha jenkinsii and Their Biological Activities
Abstract
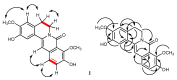
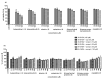
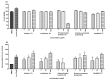
The phytochemical investigation of Huberantha jenkinsii resulted in the isolation of two new and five known compounds. The new compounds were characterized as undescribed 8-oxoprotoberberine alkaloids and named huberanthines A and B, whereas the known compounds were identified as allantoin, oxylopinine, N-trans-feruloyl tyramine, N-trans-p-coumaroyl tyramine, and mangiferin. The structure determination was accomplished by spectroscopic methods. To evaluate therapeutic potential in diabetes and Parkinson's disease, the isolates were subjected to assays for their α-glucosidase inhibitory activity, cellular glucose uptake stimulatory activity, and protective activity against neurotoxicity induced by 6-hydroxydopamine (6-OHDA). The results suggested that mangiferin was the most promising lead compound, demonstrating significant activity in all the test systems.
Keywords: Huberantha jenkinsii; Parkinsonism; diabetes; glucose uptake; α-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
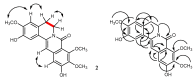
References
-
- Global Status Report on Noncommunicable Diseases 2010. World Health Organization; Geneva, Switzerland: 2011. pp. 33–40.
-
- Furman B.L., Candasamy M., Bhattamisra S.K., Veettil S.K. Reduction of blood glucose by plant extracts and their use in the treatment of diabetes mellitus; discrepancies in effectiveness between animal and human studies. J. Ethnopharmacol. 2019;247:112264. doi: 10.1016/j.jep.2019.112264. - DOI - PubMed
-
- Tadtong S., Chatsumpun N., Sritularak B., Jongbunprasert V., Ploypradith P., Likhitwitayawuid K. Effects of oxyresveratrol and its derivatives on cultured P19-derived neurons. Trop. J. Pharm. Res. 2017;15:2619. doi: 10.4314/tjpr.v15i12.12. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

