Lytic Induction Therapy against Epstein-Barr Virus-Associated Malignancies: Past, Present, and Future
- PMID: 32748879
- PMCID: PMC7465660
- DOI: 10.3390/cancers12082142
Lytic Induction Therapy against Epstein-Barr Virus-Associated Malignancies: Past, Present, and Future
Abstract
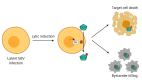
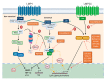
Epstein-Barr virus (EBV) lytic induction therapy is an emerging virus-targeted therapeutic approach that exploits the presence of EBV in tumor cells to confer specific killing effects against EBV-associated malignancies. Efforts have been made in the past years to uncover the mechanisms of EBV latent-lytic switch and discover different classes of chemical compounds that can reactivate the EBV lytic cycle. Despite the growing list of compounds showing potential to be used in the lytic induction therapy, only a few are being tested in clinical trials, with varying degrees of success. This review will summarize the current knowledge on EBV lytic reactivation, the major hurdles of translating the lytic induction therapy into clinical settings, and highlight some potential strategies in the future development of this therapy for EBV-related lymphoid and epithelial malignancies.
Keywords: EBV-associated gastric carcinoma; Epstein–Barr virus; Hodgkin lymphoma; T-/NK-/B-cell non-Hodgkin lymphoma; endemic Burkitt lymphoma; lytic induction therapy; nasopharyngeal carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hui K.F., Chan T.F., Yang W., Shen J.J., Lam K.P., Kwok H., Sham P.C., Tsao S.W., Kwong D.L., Lung M.L., et al. High risk Epstein-Barr virus variants characterized by distinct polymorphisms in the EBER locus are strongly associated with nasopharyngeal carcinoma. Int. J. Cancer. 2019;144:3031–3042. doi: 10.1002/ijc.32049. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

