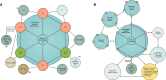
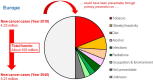
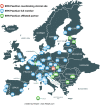
Towards a cancer mission in Horizon Europe: recommendations
- PMID: 32749074
- PMCID: PMC7400777
- DOI: 10.1002/1878-0261.12763
Towards a cancer mission in Horizon Europe: recommendations
Abstract
A comprehensive translational cancer research approach focused on personalized and precision medicine, and covering the entire cancer research-care-prevention continuum has the potential to achieve in 2030 a 10-year cancer-specific survival for 75% of patients diagnosed in European Union (EU) member states with a well-developed healthcare system. Concerted actions across this continuum that spans from basic and preclinical research through clinical and prevention research to outcomes research, along with the establishment of interconnected high-quality infrastructures for translational research, clinical and prevention trials and outcomes research, will ensure that science-driven and social innovations benefit patients and individuals at risk across the EU. European infrastructures involving comprehensive cancer centres (CCCs) and CCC-like entities will provide researchers with access to the required critical mass of patients, biological materials and technological resources and can bridge research with healthcare systems. Here, we prioritize research areas to ensure a balanced research portfolio and provide recommendations for achieving key targets. Meeting these targets will require harmonization of EU and national priorities and policies, improved research coordination at the national, regional and EU level and increasingly efficient and flexible funding mechanisms. Long-term support by the EU and commitment of Member States to specialized schemes are also needed for the establishment and sustainability of trans-border infrastructures and networks. In addition to effectively engaging policymakers, all relevant stakeholders within the entire continuum should consensually inform policy through evidence-based advice.
Keywords: European healthcare systems; cancer mission; cancer research/care/prevention continuum; comprehensive cancer centres; patient empowerment; science policy.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
In the past 5 years, Dr. Baumann attended an advisory board meeting of MERCK KGaA (Darmstadt), for which the University of Dresden received a travel grant. He further received funding for his research projects and for educational grants to the University of Dresden by Teutopharma GmbH (2011–2015), IBA (2016), Bayer AG (2016–2018), Merck KGaA (2014–2030), Medipan GmbH (2014–2018). For the German Cancer Research Centre (DKFZ, Heidelberg), Dr. Baumann is on the supervisory boards of HI‐STEM gGmbH (Heidelberg) and is also member of the supervisory body of the Charité University Hospital, Berlin. Dr. Baumann, as former chair of OncoRay (Dresden) and present CEO and Scientific Chair of the German Cancer Research Centre (DKFZ, Heidelberg), was or is responsible for collaborations with a multitude of companies and institutions, worldwide. In this capacity, he has signed/signs contracts for his institute(s) and for the staff for research funding and/or collaborations with industry and academia, worldwide, including but not limited to pharmaceutical corporations like Bayer, Boehringer Ingelheim, Bosch, Roche and other corporations like Siemens, IBA, Varian, Elekta, Bruker and others. In this role, he was/is further responsible for commercial technology transfer activities of his institute(s), including the DKFZ‐PSMA617 related patent portfolio [WO2015055318 (A1), ANTIGEN (PSMA)] and similar IP portfolios. Dr. Baumann confirms that none of the above funding sources was involved in the preparation of this paper.
Carlos Caldas is a member of AstraZeneca's iMED External Science Panel, a member of Illumina's Scientific Advisory Board and a recipient of research grants (administered by the University of Cambridge) from Genentech, Roche, AstraZeneca and Servier.
Outside the scope of this work, Caroline Dive declares research funding/grants received from: AstraZeneca, Astex Pharmaceuticals, Bioven, Amgen, Carrick Therapeutics, Merck AG, Taiho Oncology, GSK, Bayer, Boehringer Ingelheim, Roche, BMS, Novartis, Celgene, Epigene Therapeutics Inc, Angle PLC, Menarini, Clearbridge Biomedics; personal honoraria for consultancy and/or advisory board has been received from: Biocartis, Merck, AstraZeneca and Illumina.
Carolina Espina and Joachim Schüz state: where authors are identified as personnel of the International Agency for Research on Cancer/ World Health Organization, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/ World Health Organization.
Peter Nagy is supported by the 2019 Hungarian Thematic Excellence Program (TUDFO/51757/2019‐ITM).
Josep Tabernero reports personal financial interest in form of scientific consultancy role for Array Biopharma, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Chugai, Genentech, Inc., Genmab A/S, Halozyme, Imugene Limited, Inflection Biosciences Limited, Ipsen, Kura Oncology, Lilly, MSD, Menarini, Merck Serono, Merrimack, Merus, Molecular Partners, Novartis, Peptomyc, Pfizer, Pharmacyclics, ProteoDesign SL, Rafael Pharmaceuticals, F. Hoffmann‐La Roche Ltd, Sanofi, SeaGen, Seattle Genetics, Servier, Symphogen, Taiho, VCN Biosciences, Biocartis, Foundation Medicine, HalioDX SAS and Roche Diagnostics.
All other authors declared no conflict of interest.
Figures