Incidence of Pseudoprogression during Immune Checkpoint Inhibitor Therapy for Solid Tumors: A Systematic Review and Meta-Analysis
- PMID: 32749204
- PMCID: PMC7526949
- DOI: 10.1148/radiol.2020200443
Incidence of Pseudoprogression during Immune Checkpoint Inhibitor Therapy for Solid Tumors: A Systematic Review and Meta-Analysis
Abstract
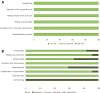
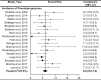
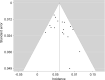
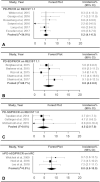
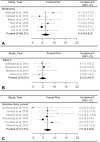
BackgroundImmune checkpoint inhibitors (ICIs) have been increasingly used in cancer treatment, and a subset of patients undergo pseudoprogression. Recognizing the incidence of pseudoprogression is critical for clinical practice.PurposeTo evaluate by systematic review and meta-analysis the incidence of pseudoprogression in cancer treatment with ICIs, and compare the incidence according to response criteria, tumor types, and immunotherapeutic agents.Materials and MethodsMedline and Embase were searched to identify relevant studies published before December 31, 2018. Clinical trials, post hoc analysis of clinical trials, and prospective studies on ICI treatment in patients with malignant solid tumors were included. Pooled incidence of pseudoprogression for all included studies, per definition of pseudoprogression, cancer type, and drug type, was obtained by random-effects models with inverse variance weighting model.ResultsSeventeen studies with 3402 patients were analyzed. The pooled incidence of pseudoprogression was 6.0% (95% confidence interval: 5.0%, 7.0%). The definition of pseudoprogression were divided into four categories: progressive disease followed by partial response (PR) or complete response (CR) but not stable disease (SD) with Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (six studies); progressive disease followed by SD or PR or CR with RECIST 1.1 (five studies); progressive disease followed by SD or PR or CR with RECIST 1.0 (three studies); and progressive disease followed by SD or PR or CR with immune-related response criteria (irRC) (three studies). Incidence of pseudoprogression varied from 4.5% to 8.0% per definition, ranged from 5.0% to 7.0% per cancer type, and was 5.6% with the monotherapy of programmed cell death-1 inhibitor.ConclusionThe overall incidence of pseudoprogression was 6.0% and was less than 10% in subgroup analyses according to the definitions of pseudoprogression, cancer type, and immune checkpoint inhibitor type. Varying definitions across trials and studies indicates the need for uniform criteria of pseudoprogression for solid tumors.© RSNA, 2020Online supplemental material is available for this article.See also the article by Dodd and MacDermott in this issue.
Figures


Comment in
-
Pseudoprogression during Immune Checkpoint Inhibitor Therapy for Solid Tumors: Clarity Amidst Rapid Evolution.Radiology. 2020 Oct;297(1):97-98. doi: 10.1148/radiol.2020202842. Epub 2020 Aug 4. Radiology. 2020. PMID: 32772876 No abstract available.
References
-
- Borcoman E, Kanjanapan Y, Champiat S, et al. . Novel patterns of response under immunotherapy. Ann Oncol 2019;30(3):385–396. - PubMed
-
- Wolchok JD, Hoos A, O’Day S, et al. . Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15(23):7412–7420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

