Myocarditis associated with immune checkpoint inhibitors: Practical considerations in diagnosis and management
- PMID: 32749254
- PMCID: PMC7460679
- DOI: 10.14744/AnatolJCardiol.2020.79584
Myocarditis associated with immune checkpoint inhibitors: Practical considerations in diagnosis and management
Abstract
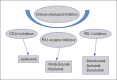
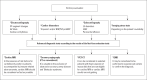
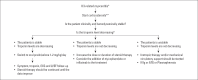
Immune checkpoint inhibitors (ICI) have caused radical changes in the treatment scheme of many types of cancer in the past 10 years. ICIs are specific monoclonal antibodies that increase T-cell mediated immune response against cancer cells. Despite important advances in cancer treatment, uncontrolled activation of cytotoxic T cells has brought along many autoimmune clinical side effects, especially acute myocarditis. Although the incidence of ICI-related myocarditis is about 1%, it is remarkable in terms of mortality rate reaching 46% and demonstrating the necessity of rapid diagnosis and multidisciplinary approach. The present review aimed to summarize the heterogeneous symptomatology of ICI-associated myocarditis, clinical presentation ranging from elevated asymptomatic cardiac enzyme levels to cardiogenic shock, prominent diagnostic value of cardiac magnetic resonance imaging, and current information on the effectiveness of immunosuppressants in therapy.
Conflict of interest statement
Figures

References
-
- Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–58. - PubMed
-
- Chen DY, Huang WK, Chien-Chia Wu V, Chang WC, Chen JS, et al. Cardiovascular toxicity of immune checkpoint inhibitors in cancer patients:A review when cardiology meets immuno-oncology. J Formos Med Assoc. 2019 S0929-6646(19)30408-5. - PubMed
-
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors:A Review. JAMA Oncol. 2016;2:1346–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

