Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease
- PMID: 32749712
- PMCID: PMC7436499
- DOI: 10.1002/jmv.26386
Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease
Abstract
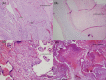
The full impact of coronavirus disease 2019 (COVID-19) on pregnancy remains uncharacterized. Current literature suggests minimal maternal, fetal, and neonatal morbidity and mortality. COVID-19 manifestations appear similar between pregnant and nonpregnant women. We present a case of placental severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus in a woman with mild COVID-19 disease, then review the literature. Reverse transcriptase polymerase chain reaction was performed to detect SARS-CoV-2. Immunohistochemistry staining was performed with specific monoclonal antibodies to detect SARS-CoV-2 antigen or to identify trophoblasts. A 29-year-old multigravida presented at 40-4/7 weeks for labor induction. With myalgias 2 days prior, she tested positive for SARS-CoV-2. We demonstrate maternal vascular malperfusion, with no fetal vascular malperfusion, as well as SARS-CoV-2 virus in chorionic villi endothelial cells, and also rarely in trophoblasts. To our knowledge, this is the first report of placental SARS-CoV-2 despite mild COVID-19 disease (no symptoms of COVID-19 aside from myalgias); patient had no fever, cough, or shortness of breath, but only myalgias and sick contacts. Despite her mild COVID-19 disease in pregnancy, we demonstrate placental vasculopathy and presence of SARS-CoV-2 virus across the placenta. Evidence of placental COVID-19 raises concern for placental vasculopathy (potentially leading to fetal growth restriction and other pregnancy complications) and possible vertical transmission-especially for pregnant women who may be exposed to COVID-19 in early pregnancy. This raises important questions of whether future pregnancy guidance should include stricter pandemic precautions, such as screening for a wider array of COVID-19 symptoms, increased antenatal surveillance, and possibly routine COVID-19 testing throughout pregnancy.
Keywords: Coronavirus; SARS coronavirus; public policy.
© 2020 Wiley Periodicals LLC.
Figures

References
-
- COVID‐19 cases in the US. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed July 18, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

