Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy
- PMID: 32749941
- PMCID: PMC7527156
- DOI: 10.1200/JCO.20.00549
Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy
Abstract
Purpose: The randomized Adjuvant Chemoradiotherapy Versus Radiotherapy Alone in Women With High-Risk Endometrial Cancer (PORTEC-3) trial investigated the benefit of combined adjuvant chemotherapy and radiotherapy (CTRT) versus radiotherapy alone (RT) for women with high-risk endometrial cancer (EC). Because The Cancer Genome Atlas defined an EC molecular classification with strong prognostic value, we investigated prognosis and impact of chemotherapy for each molecular subgroup using tissue samples from PORTEC-3 trial participants.
Methods: Paraffin-embedded tissues of 423 consenting patients were collected. Immunohistochemistry for p53 and mismatch repair (MMR) proteins, and DNA sequencing for POLE exonuclease domain were done to classify tumors as p53 abnormal (p53abn), POLE-ultramutated (POLEmut), MMR-deficient (MMRd), or no specific molecular profile (NSMP). The primary end point was recurrence-free survival (RFS). Kaplan-Meier method, log-rank test, and Cox model were used for analysis.
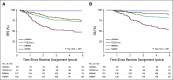
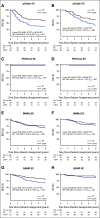
Results: Molecular analysis was successful in 410 high-risk EC (97%), identifying the 4 subgroups: p53abn EC (n = 93; 23%), POLEmut (n = 51; 12%), MMRd (n = 137; 33%), and NSMP (n = 129; 32%). Five-year RFS was 48% for patients with p53abn EC, 98% for POLEmut EC, 72% for MMRd EC, and 74% for NSMP EC (P < .001). The 5-year RFS with CTRT versus RT for p53abn EC was 59% versus 36% (P = .019); 100% versus 97% for patients with POLEmut EC (P = .637); 68% versus 76% (P = .428) for MMRd EC; and 80% versus 68% (P = .243) for NSMP EC.
Conclusion: Molecular classification has strong prognostic value in high-risk EC, with significantly improved RFS with adjuvant CTRT for p53abn tumors, regardless of histologic type. Patients with POLEmut EC had an excellent RFS in both trial arms. EC molecular classification should be incorporated in the risk stratification of these patients as well as in future trials to target specific subgroups of patients.
Trial registration: ClinicalTrials.gov NCT00411138.
Figures

References
-
- Stelloo E, Nout RA, Osse EM, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res. 2016;22:4215–4224. - PubMed
-
- Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123:802–813. - PubMed
-
- Kommoss S, McConechy MK, Kommoss F, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol. 2018;29:1180–1188. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

