The immune system as a target for therapy of SARS-CoV-2: A systematic review of the current immunotherapies for COVID-19
- PMID: 32750438
- PMCID: PMC7395832
- DOI: 10.1016/j.lfs.2020.118185
The immune system as a target for therapy of SARS-CoV-2: A systematic review of the current immunotherapies for COVID-19
Abstract
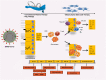
Aims: The immune response is essential for the control and resolution of viral infections. Following the outbreak of novel coronavirus disease (COVID-19), several immunotherapies were applied to modulate the immune responses of the affected patients. In this review, we aimed to describe the role of the immune system in response to COVID-19. We also provide a systematic review to collate and describe all published reports of the using immunotherapies, including convalescent plasma therapy, monoclonal antibodies, cytokine therapy, mesenchymal stem cell therapy, and intravenous immunoglobulin and their important outcomes in COVID-19 patients.
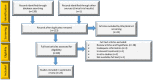
Material and methods: A thorough search strategy was applied to identify published research trials in PubMed, Scopus, Medline, and EMBASE from Dec 1, 2019, to May 4, 2020, for studies reporting clinical outcomes of COVID-19 patients treated with immunotherapies along with other standard cares.
Key findings: From an initial screen of 80 identified studies, 24 studies provided clinical outcome data on the use of immunotherapies for the treatment of COVID-19 patients, including convalescent plasma therapy (33 patients), monoclonal antibodies (55 patients), interferon (31 patients), mesenchymal stem cell therapy (8 patient), and immunoglobulin (63 patients). Except for nine severe patients who died after treatment, most patients were recovered from COVID-19 with improved clinical symptoms and laboratory assessment.
Significance: Based on the available evidence, it seems that treatment with immunotherapy along with other standard cares could be an effective and safe approach to modulate the immune system and improvement of clinical outcomes.
Keywords: COVID-19; Coronavirus; Immune system; Immunotherapy; SARS-COV-2.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that there are no conflicts of interest.
Figures


References
-
- Abdulamir A.S., Hafidh R.R. The possible immunological pathways for the variable immunopathogenesis of COVID-19 infections among healthy adults, elderly and children. Electron J.Gen. Med. 2020;17 doi: 10.29333/ejgm/7850. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

