Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients
- PMID: 32750442
- PMCID: PMC7395653
- DOI: 10.1016/j.jhep.2020.07.040
Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients
Abstract
Background & aims: The incidence and outcomes of coronavirus disease 2019 (COVID-19) in immunocompromised patients are a matter of debate.
Methods: We performed a prospective nationwide study including a consecutive cohort of liver transplant patients with COVID-19 recruited during the Spanish outbreak from 28 February to 7 April, 2020. The primary outcome was severe COVID-19, defined as the need for mechanical ventilation, intensive care, and/or death. Age- and gender-standardised incidence and mortality ratios (SIR and SMR) were calculated using data from the Ministry of Health and the Spanish liver transplant registry. Independent predictors of severe COVID-19 among hospitalised patients were analysed using multivariate Cox regression.
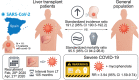
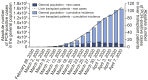
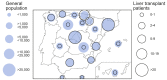
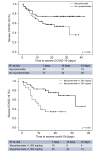
Results: A total of 111 liver transplant patients were diagnosed with COVID-19 (SIR = 191.2 [95% CI 190.3-192.2]). The epidemiological curve and geographic distribution overlapped widely between the liver transplant and general populations. After a median follow-up of 23 days, 96 patients (86.5%) were admitted to hospital and 22 patients (19.8%) required respiratory support. A total of 12 patients were admitted to the ICU (10.8%). The mortality rate was 18%, which was lower than in the matched general population (SMR = 95.5 [95% CI 94.2-96.8]). Overall, 35 patients (31.5%) met criteria of severe COVID-19. Baseline immunosuppression containing mycophenolate was an independent predictor of severe COVID-19 (relative risk = 3.94; 95% CI 1.59-9.74; p = 0.003), particularly at doses higher than 1,000 mg/day (p = 0.003). This deleterious effect was not observed with calcineurin inhibitors or everolimus and complete immunosuppression withdrawal showed no benefit.
Conclusions: Being chronically immunosuppressed, liver transplant patients have an increased risk of acquiring COVID-19 but their mortality rates are lower than the matched general population. Upon hospital admission, mycophenolate dose reduction or withdrawal could help in preventing severe COVID-19. However, complete immunosuppression withdrawal should be discouraged.
Lay summary: In liver transplant patients, chronic immunosuppression increases the risk of acquiring COVID-19 but it could reduce disease severity. Complete immunosuppression withdrawal may not be justified. However, mycophenolate withdrawal or temporary conversion to calcineurin inhibitors or everolimus until disease resolution could be beneficial in hospitalised patients.
Keywords: COVID-19; Calcineurin inhibitors; Epidemiology; Everolimus; Immunosuppression; Mycophenolate; Pneumonia; SARS-CoV-2; Standardised incidence; Standardised mortality; Tacrolimus; Transplantation.
Copyright © 2020 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest JC has received lecture fees from Chiesi, Astellas, and Novartis and is an advisory to Chiesi. MR-P has received lecture fees from Astellas, Novartis, and Intercept Pharma. MS has received lecture fees from Astellas, Novartis, and Chiesi and is an advisory to Jazz and Novartis. MG has received speaker fees from and/or acted as advisor for Astellas, Novartis, Chiesi, Baxter, and Medtronic. MN has received lecture fees from Astellas Pharma. MI has received lecture fees from BMS. CV has received lecture fees from Novartis, Abbie, and Gilead. AC has received lecture fees from Astellas Pharma. GB-F has received lecture fees from Bayer and Astellas. JLM has received lecture fees from Bayer and Gilead. JP has received lecture fees by Astellas, Chiesi, and Gilead. AA-M, AM-S, JG, JN, JB-S, AC, LL, AC, AF-Y, CL, IF, CF, LC, SP, PR, MG-D, RG-G, LH, FN, AO, JA, EF, FG-P, ST and GDR have no conflict of interest to disclose regarding this manuscript. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Comment in
-
Age and comorbidity are central to the risk of death from COVID-19 in liver transplant recipients.J Hepatol. 2021 Jul;75(1):226-228. doi: 10.1016/j.jhep.2021.01.036. Epub 2021 Feb 5. J Hepatol. 2021. PMID: 33556419 Free PMC article. No abstract available.
References
-
- Ministerio de Sanidad, Gobierno de España https://www.mscbs.gob.es/en/profesionales/saludPublica/ccayes/alertasAct... Available at.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

