Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: predictive biomarkers and clinical outcomes
- PMID: 32750704
- PMCID: PMC7850044
- DOI: 10.1093/neuonc/noaa183
Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: predictive biomarkers and clinical outcomes
Abstract
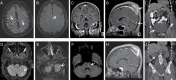
Background: CD19-directed chimeric antigen receptor (CAR) T-cell therapy (CAR-T) has emerged as effective for relapsed/refractory large B-cell lymphoma (R/R LBCL). The neurologic toxicity seen with CAR-T, referred to as immune effector cell-associated neurotoxicity syndrome (ICANS), is poorly understood. To better elucidate the clinical characteristics, treatment outcomes, and correlative biomarkers of ICANS, we review here a single-center analysis of ICANS after CAR T-cell therapy in R/R LBCL.
Methods: Patients (n = 45) with R/R LBCL treated with axicabtagene ciloleucel (axi-cel) were identified. Data regarding treatment course, clinical outcomes, and correlative studies were collected. Patients were monitored and graded for ICANS via CARTOX-10 scoring and Common Terminology Criteria for Adverse Events (CTCAE) v4.03 criteria, respectively.
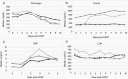
Results: Twenty-five (56%) patients developed ICANS, 18 (72%) of whom had severe (CTCAE grades 3-4) ICANS. Median time to development of ICANS was 5 days (range, 3-11). Elevated pre-infusion (day 0 [D0]) fibrinogen (517 vs 403 mg/dL, upper limit of normal [ULN] 438 mg/dL, P = 0.01) and D0 lactate dehydrogenase (618 vs 506 units/L, ULN 618 units/L, P = 0.04) were associated with ICANS. A larger drop in fibrinogen was associated with ICANS (393 vs 200, P < 0.01). Development of ICANS of any grade had no effect on complete remission (CR), progression-free survival (PFS), or overall survival (OS). Duration and total dose of steroid treatment administered for ICANS did not influence CR, PFS, or OS.
Conclusions: ICANS after CAR-T with axi-cel for R/R LBCL was seen in about half of patients, the majority of which were high grade. Contrary to previous reports, neither development of ICANS nor its treatment were associated with inferior CR, PFS, or OS. The novel finding of high D0 fibrinogen level can identify patients at higher risk for ICANS.
Keywords: chimeric antigen receptor T cells; fibrinogen; immunotherapy; lymphoma; neurotoxicity.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Immune effector cell-associated acute stroke: A novel entity?Eur J Cancer. 2023 Nov;194:113352. doi: 10.1016/j.ejca.2023.113352. Epub 2023 Sep 29. Eur J Cancer. 2023. PMID: 37852043 No abstract available.
References
-
- Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
-
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. - PubMed

