Investigation on the metabolic characteristics of isobavachin in Psoralea corylifolia L. (Bu-gu-zhi) and its potential inhibition against human cytochrome P450s and UDP-glucuronosyltransferases
- PMID: 32750744
- PMCID: PMC8861878
- DOI: 10.1111/jphp.13337
Investigation on the metabolic characteristics of isobavachin in Psoralea corylifolia L. (Bu-gu-zhi) and its potential inhibition against human cytochrome P450s and UDP-glucuronosyltransferases
Abstract
Objectives: Isobavachin is a phenolic with anti-osteoporosis activity. This study aimed to explore its metabolic fates in vivo and in vitro, and to investigate the potential drug-drug interactions involving CYPs and UGTs.
Methods: Metabolites of isobavachin in mice were first identified and characterized. Oxidation and glucuronidation study were performed using liver and intestine microsomes. Reaction phenotyping, activity correlation analysis and relative activity factor approaches were employed to identify the main CYPs and UGTs involved in isobavachin metabolism. Through kinetic modelling, inhibition mechanisms towards CYPs and UGTs were also explored.
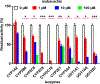
Key findings: Two glucuronides (G1 - G2) and three oxidated metabolites (M1 - M3) were identified in mice. Additionally, isobavachin underwent efficient oxidation and glucuronidation by human liver microsomes and HIM with CLint values from 5.53 to 148.79 μl/min per mg. CYP1A2, 2C19 contributed 11.3% and 17.1% to hepatic metabolism of isobavachin, respectively, with CLint values from 8.75 to 77.33 μl/min per mg. UGT1As displayed CLint values from 10.73 to 202.62 μl/min per mg for glucuronidation. Besides, significant correlation analysis also proved that CYP1A2, 2C19 and UGT1A1, 1A9 were main contributors for the metabolism of isobavachin. Furthermore, mice may be the appropriate animal model for predicting its metabolism in human. Moreover, isobavachin exhibited broad inhibition against CYP2B6, 2C9, 2C19, UGT1A1, 1A9, 2B7 with Ki values from 0.05 to 3.05 μm.
Conclusions: CYP1A2, 2C19 and UGT1As play an important role in isobavachin metabolism. Isobavachin demonstrated broad-spectrum inhibition of CYPs and UGTs.
Keywords: CYPs; UGTs; drug-drug interactions; isobavachin; metabolism; mice.
© 2020 Royal Pharmaceutical Society.
Conflict of interest statement
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Figures

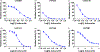


References
-
- Chopra B et al. Psoralea corylifolia L. (Buguchi)-Folklore to modern evidence: Review. Fitoterapia 2019; 90: 44–56. - PubMed
-
- Li WD et al. Osteoblasts proliferation and differentiation stimulating activities of the main components of Fructus Psoraleae corylifoliae. Phytomedicine 2014; 21: 400–405. - PubMed
-
- Watjen W et al. Prenylation enhances cytotoxicity of apigenin and liquiritigenin in rat H4IIE hepatoma and C6 glioma cells. Food Chem Toxicol 2007; 45: 119–124. - PubMed
MeSH terms
Substances
Grants and funding
- 81903704/National Natural Science Foundation of China
- ZIA BC005708/ImNIH/Intramural NIH HHS/United States
- SIMM1903KF-07/State Key Laboratory of Drug Research
- 20A350012/Foundation of He'nan Educational Committee
- 81630097/State Key Program of National Natural Science Foundation of China
- 81220108028/Major Project for International Cooperation and Exchange of the National Natural Science Foundation of China
- 81703799/National Natural Science Foundation of China
- B13038/Program of Introducing Talents of Discipline to Universities
- 81803638/National Natural Science Foundation of China
- 2019A1515011285/Guangdong Basic and Applied Basic Research Foundation

