Structure-Antifouling Activity Relationship and Molecular Targets of Bio-Inspired(thio)xanthones
- PMID: 32751491
- PMCID: PMC7463931
- DOI: 10.3390/biom10081126
Structure-Antifouling Activity Relationship and Molecular Targets of Bio-Inspired(thio)xanthones
Abstract
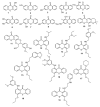
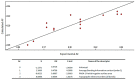
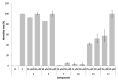
The development of alternative ecological and effective antifouling technologies is still challenging. Synthesis of nature-inspired compounds has been exploited, given the potential to assure commercial supplies of potential ecofriendly antifouling agents. In this direction, the antifouling activity of a series of nineteen synthetic small molecules, with chemical similarities with natural products, were exploited in this work. Six (4, 5, 7, 10, 15 and 17) of the tested xanthones showed in vivo activity toward the settlement of Mytilus galloprovincialis larvae (EC50: 3.53-28.60 µM) and low toxicity to this macrofouling species (LC50 > 500 µM and LC50/EC50: 17.42-141.64), and two of them (7 and 10) showed no general marine ecotoxicity (<10% of Artemia salina mortality) after 48 h of exposure. Regarding the mechanism of action in mussel larvae, the best performance compounds 4 and 5 might be acting by the inhibition of acetylcholinesterase activity (in vitro and in silico studies), while 7 and 10 showed specific targets (proteomic studies) directly related with the mussel adhesive structure (byssal threads), given by the alterations in the expression of Mytilus collagen proteins (PreCols) and proximal thread proteins (TMPs). A quantitative structure-activity relationship (QSAR) model was built with predictive capacity to enable speeding the design of new potential active compounds.
Keywords: antifouling activity; chemical synthesis; invertebrates; molecular targets; xanthones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Galil B.S., McKenzie C., Bailey S., Campbell M., Davidson I., Drake L., Hewitt C., Occhipinti-Ambrogi A., Piola R. ICES Viewpoint background document: Evaluating and mitigating introduction of marine non-native species via vessel bio- fouling. ICES Ad Hoc Rep. 2019;2019:17.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

