Functional Alterations in the Olfactory Neuronal Circuit Occur before Hippocampal Plasticity Deficits in the P301S Mouse Model of Tauopathy: Implications for Early Diagnosis and Translational Research in Alzheimer's Disease
- PMID: 32751531
- PMCID: PMC7432464
- DOI: 10.3390/ijms21155431
Functional Alterations in the Olfactory Neuronal Circuit Occur before Hippocampal Plasticity Deficits in the P301S Mouse Model of Tauopathy: Implications for Early Diagnosis and Translational Research in Alzheimer's Disease
Abstract
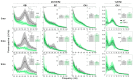
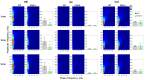
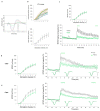
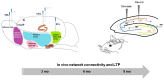
Alzheimer's disease (AD) is characterized by neuronal loss and impaired synaptic transmission, ultimately leading to cognitive deficits. Early in the disease, the olfactory track seems most sensitive to tauopathy, while most plasticity studies focused on the hippocampal circuits. Functional network connectivity (FC) and long-term potentiation (LTP), considered as the plasticity substrate of learning and memory, were longitudinally assessed in mice of the P301S model of tauopathy following the course (time and location) of progressively neurodegenerative pathology (i.e., at 3, 6, and 9 months of age) and in their wild type (WT) littermates. Using in vivo local field potential (LFP) recordings, early (at three months) dampening in the gamma oscillatory activity and impairments in the phase-amplitude theta-gamma coupling (PAC) were found in the olfactory bulb (OB) circuit of P301S mice, which were maintained through the whole course of pathology development. In contrast, LFP oscillatory activity and PAC indices were normal in the entorhinal cortex, hippocampal CA1 and CA3 nuclei. Field excitatory postsynaptic potential (fEPSP) recordings from the Shaffer collateral (SC)-CA1 hippocampal stratum pyramidal revealed a significant altered synaptic LTP response to high-frequency stimulation (HFS): at three months of age, no significant difference between genotypes was found in basal synaptic activity, while signs of a deficit in short term plasticity were revealed by alterations in the fEPSPs. At six months of age, a slight deviance was found in basal synaptic activity and significant differences were observed in the LTP response. The alterations in network oscillations at the OB level and impairments in the functioning of the SC-CA1 pyramidal synapses strongly suggest that the progression of tau pathology elicited a brain area, activity-dependent disturbance in functional synaptic transmission. These findings point to early major alterations of neuronal activity in the OB circuit prior to the disturbance of hippocampal synaptic plasticity, possibly involving tauopathy in the anomalous FC. Further research should determine whether those early deficits in the OB network oscillations and FC are possible mechanisms that potentially promote the emergence of hippocampal synaptic impairments during the progression of tauopathy.
Keywords: Alzheimer’s disease; LTP; neural oscillations; olfactory neural network; tau; translational marker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Shaw L.M., Vanderstichele H., Knapik-Czajka M., Figurski M., Coart E., Blennow K., Soares H., Simon A.J., Lewczuk P., Dean R.A., et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121:597–609. doi: 10.1007/s00401-011-0808-0. - DOI - PMC - PubMed
-
- Clark C.M., Xie S., Chittams J., Ewbank D., Peskind E., Galasko D., Morris J.C., McKeel D.W., Farlow M., Weitlauf S.L., et al. Cerebrospinal fluid tau and beta-amyloid: How well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch. Neurol. 2003;60:1696–1702. doi: 10.1001/archneur.60.12.1696. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

