Point-of-Care Diagnostics in Coagulation Management
- PMID: 32751629
- PMCID: PMC7435714
- DOI: 10.3390/s20154254
Point-of-Care Diagnostics in Coagulation Management
Abstract
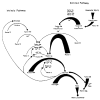
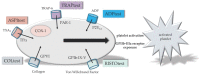
This review provides a comprehensive and up-to-date overview of point-of-care (POC) devices most commonly used for coagulation analyses in the acute settings. Fast and reliable assessment of hemostasis is essential for the management of trauma and other bleeding patients. Routine coagulation assays are not designed to visualize the process of clot formation, and their results are obtained only after 30-90 m due to the requirements of sample preparation and the analytical process. POC devices such as viscoelastic coagulation tests, platelet function tests, blood gas analysis and other coagulometers provide new options for the assessment of hemostasis, and are important tools for an individualized, goal-directed, and factor-based substitution therapy. We give a detailed overview of the related tests, their characteristics and clinical implications. This review emphasizes the evident advantages of the speed and predictive power of POC clot measurement in the context of a goal-directed and algorithm-based therapy to improve the patient's outcome. Interpretation of viscoelastic tests is facilitated by a new visualization technology.
Keywords: ROTEM®; coagulation management; hemorrhage; platelet function test; point-of-care systems.
Conflict of interest statement
S.D.S. and J.R. have no conflict of interest to declare. J.-D.S. received lecture and advisory honoraria from Bayer (Switzerland), Shire/Takeda (Switzerland), Sanofi (Switzerland), and Siemens Healthineers (Switzerland). A.K. received honoraria for lecturing from Bayer AG Switzerland. The University of Zurich and Instrumentation Laboratory Company/Werfen Corporation, Bedford, MA, USA, signed a letter of intent regarding a joint development and licensing agreement to develop a product based on the concept of Visual Clot. Within the framework of this letter of intent and a potential later agreement, D.W.T. and D.R.S. might receive royalties as designated inventors. The University of Zurich and Philips Medizin Systeme Böblingen GmbH, Böblingen, Germany and Konikljike Philips N.V., Amsterdam, The Netherlands signed a joint development and licensing agreement to develop a product based on the concept of Visual Patient. Within the framework of this agreement, D.W.T. might receive royalties as designated inventor. D.W.T. received travel support for consulting Instrumentation Laboratory, Bedford, MA, USA and consults Philips Research/Philips Electronics Nederland B.V., Eindhoven, The Netherlands. Dr. Spahn’s academic department is receiving grant support from the Swiss National Science Foundation, Berne, Switzerland, the Swiss Society of Anesthesiology and Reanimation (SGAR), Berne, Switzerland, the Swiss Foundation for Anesthesia Research, Zurich, Switzerland, Vifor SA, Villars-sur-Glâne, Switzerland. Dr. Spahn is co-chair of the ABC-Trauma Faculty, sponsored by unrestricted educational grants from Novo Nordisk Health Care AG, Zurich, Switzerland, CSL Behring GmbH, Marburg, Germany, LFB Biomédicaments, Courtaboeuf Cedex, France and Octapharma AG, Lachen, Switzerland. Dr. Spahn received honoraria/travel support for consulting or lecturing from: Danube University of Krems, Austria, US Department of Defense, Washington, USA, European Society of Anesthesiology, Brussels, BE, Korean Society for Patient Blood Management, Seoul, Korea, Korean Society of Anesthesiologists, Seoul, Korea, Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, Paris, France, Baxalta Switzerland AG, Volketswil, Switzerland, Bayer AG, Zürich, Switzerland, B. Braun Melsungen AG, Melsungen, Germany, Boehringer Ingelheim GmbH, Basel, Switzerland, Bristol-Myers-Squibb, Rueil-Malmaison Cedex, France and Baar, Switzerland, CSL Behring GmbH, Hattersheim am Main, Germany and Berne, Switzerland, Celgene International II Sàrl, Couvet, Switzerland, Daiichi Sankyo AG, Thalwil, Switzerland, Ethicon Sàrl, Neuchâtel, Switzerland, Haemonetics, Braintree, MA, USA, Instrumentation Laboratory (Werfen), Bedford, MA, USA, LFB Biomédicaments, Courtaboeuf Cedex, France, Merck Sharp & Dohme, Kenilworth, New Jersey, USA, PAION Deutschland GmbH, Aachen, Germany, Pharmacosmos A/S, Holbaek, Denmark, Photonics Healthcare B.V., Utrecht, Netherlands, Pfizer AG, Zürich, Switzerland, Pierre Fabre Pharma, Alschwil, Switzerland, Roche Diagnostics International Ltd., Reinach, Switzerland, Sarstedt AG & Co., Sevelen, Switzerland and Nümbrecht, Germany, Shire Switzerland GmbH, Zug, Switzerland, Tem International GmbH, Munich, Germany, Vifor Pharma, Munich, Germany, Neuilly sur Seine, France and Villars-sur-Glâne, Switzerland, Vifor (International) AG, St. Gallen, Switzerland, Zuellig Pharma Holdings, Singapore, Singapore.
Figures




References
-
- Spahn D.R., Bouillon B., Cerny V., Duranteau J., Filipescu D., Hunt B.J., Komadina R., Maegele M., Nardi G., Riddez L., et al. The european guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care. 2019;23:98. doi: 10.1186/s13054-019-2347-3. - DOI - PMC - PubMed
-
- Theusinger O.M., Wanner G.A., Emmert M.Y., Billeter A., Eismon J., Seifert B., Simmen H.P., Spahn D.R., Baulig W. Hyperfibrinolysis diagnosed by rotational thromboelastometry (rotem) is associated with higher mortality in patients with severe trauma. Anesth. Analg. 2011;113:1003–1012. doi: 10.1213/ANE.0b013e31822e183f. - DOI - PubMed
-
- Gonzalez E., Moore E.E., Moore H.B., Chapman M.P., Chin T.L., Ghasabyan A., Wohlauer M.V., Barnett C.C., Bensard D.D., Biffl W.L., et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: A pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann. Surg. 2016;263:1051–1059. doi: 10.1097/SLA.0000000000001608. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

