Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials
- PMID: 32751652
- PMCID: PMC7552663
- DOI: 10.3390/bioengineering7030083
Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials
Abstract
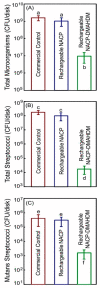
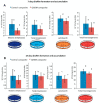
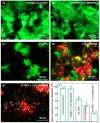
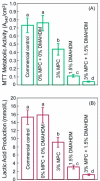
Polymeric materials are the first choice for restoring tooth cavities, bonding tooth-colored fillings, sealing root canal systems, and many other dental restorative applications. However, polymeric materials are highly susceptible to bacterial attachment and colonization, leading to dental diseases. Many approaches have been investigated to minimize the formation of biofilms over polymeric restorative materials and at the tooth/material interfaces. Among them, contact-killing compounds have shown promising results to inhibit dental biofilms. Contact-killing compounds can be immobilized within the polymer structure, delivering a long-lasting effect with no leaching or release, thus providing advantages compared to release-based materials. This review discusses cutting-edge research on the development of contact-killing compounds in dental restorative materials to target oral pathogens. Contact-killing compounds in resin composite restorations, dental adhesives, root canal sealers, denture-based materials, and crown cements have all demonstrated promising antibacterial properties. Contact-killing restorative materials have been found to effectively inhibit the growth and activities of several oral pathogens related to dental caries, periodontal diseases, endodontic, and fungal infections. Further laboratory optimization and clinical trials using translational models are needed to confirm the clinical applicability of this new generation of contact-killing dental restorative materials.
Keywords: antibacterial agents; biofilms; composite resins; dental caries; polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Dental Caries Among Adults and Older Adults. [(accessed on 8 June 2020)]; Available online: https://www.cdc.gov/oralhealth/publications/OHSR-2019-dental-carries-adu....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

