Nucleocapsid Structure of Negative Strand RNA Virus
- PMID: 32751700
- PMCID: PMC7472042
- DOI: 10.3390/v12080835
Nucleocapsid Structure of Negative Strand RNA Virus
Abstract
Negative strand RNA viruses (NSVs) include many important human pathogens, such as influenza virus, Ebola virus, and rabies virus. One of the unique characteristics that NSVs share is the assembly of the nucleocapsid and its role in viral RNA synthesis. In NSVs, the single strand RNA genome is encapsidated in the linear nucleocapsid throughout the viral replication cycle. Subunits of the nucleocapsid protein are parallelly aligned along the RNA genome that is sandwiched between two domains composed of conserved helix motifs. The viral RNA-dependent-RNA polymerase (vRdRp) must recognize the protein-RNA complex of the nucleocapsid and unveil the protected genomic RNA in order to initiate viral RNA synthesis. In addition, vRdRp must continuously translocate along the protein-RNA complex during elongation in viral RNA synthesis. This unique mechanism of viral RNA synthesis suggests that the nucleocapsid may play a regulatory role during NSV replication.
Keywords: 5H+3H; capsid protein motif; cofactor; cross subunit interactions; negative strand RNA virus; nucleocapsid; viral RNA-dependent RNA polymerase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
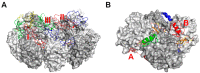
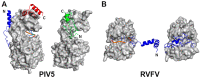
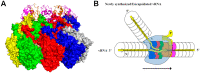
References
-
- Knipe D., Howley P., Fields B.N., Griffin D.E. Fields’ Virilogy: Principles of Virus Structure. Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2001. pp. 53–86.
-
- Dong S., Yang P., Li G., Liu B., Wang W., Liu X., Xia B., Yang C., Lou Z., Guo Y., et al. Insight into the Ebola virus nucleocapsid assembly mechanism: Crystal structure of Ebola virus nucleoprotein core domain at 1.8 A resolution. Protein Cell. 2015;6:351–362. doi: 10.1007/s13238-015-0163-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

