Aquilariae Lignum Methylene Chloride Fraction Attenuates IL-1β-Driven Neuroinflammation in BV2 Microglial Cells
- PMID: 32751738
- PMCID: PMC7432889
- DOI: 10.3390/ijms21155465
Aquilariae Lignum Methylene Chloride Fraction Attenuates IL-1β-Driven Neuroinflammation in BV2 Microglial Cells
Abstract
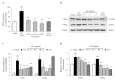
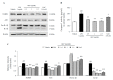
Microglial hyperactivation and neuroinflammation are known to induce neuronal death, which is one of the main causes of neurodegenerative disorders. We previously found that Aquilariae Lignum extract attenuated both neuronal excitotoxicity and neuroinflammation in vivo and in vitro. For further analysis, we extracted the methylene chloride fraction of Aquilariae Lignum to determine the bioactive compounds. In this study, we investigated the anti-neuroinflammatory effects and underlying mechanisms of the Aquilariae Lignum fraction (ALF) using lipopolysaccharide (LPS)-stimulated BV2 microglial cells. BV2 cells were pretreated with ALF (0.5, 1, and 2.5 μg/mL) before treatment with LPS (1 μg/mL). Pretreatment with ALF significantly attenuated the LPS-induced overproductions of nitric oxide (NO), cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), and interleukin (IL)-1β. These anti-inflammatory effects were supported by ALF-mediated modulation of the nuclear factor-kappa B (NF-κB) pathway. Furthermore, ALF exerted strong anti-inflammasome effects, as shown by IL-1β-specific inhibitory activity, but not activity against tumor necrosis factor (TNF)-α, along with inhibition of caspase-1 activity and NACHT, LRR, and PYD domain-containing protein 3 (NLRP3)-related molecules. These results indicate the potent anti-neuroinflammatory activity of ALF and that its underlying mechanism may involve the regulation of NLRP3 inflammasome-derived neuroinflammation in microglial cells.
Keywords: Aquilariae Lignum; NLRP3 inflammasome; microglia; neuroinflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Inhibitory effect of 9-hydroxy-6,7-dimethoxydalbergiquinol from Dalbergia odorifera on the NF-κB-related neuroinflammatory response in lipopolysaccharide-stimulated mouse BV2 microglial cells is mediated by heme oxygenase-1.Int Immunopharmacol. 2013 Nov;17(3):828-35. doi: 10.1016/j.intimp.2013.08.024. Epub 2013 Sep 19. Int Immunopharmacol. 2013. PMID: 24055019
-
Micheliolide suppresses LPS-induced neuroinflammatory responses.PLoS One. 2017 Oct 17;12(10):e0186592. doi: 10.1371/journal.pone.0186592. eCollection 2017. PLoS One. 2017. PMID: 29040306 Free PMC article.
-
Anti-neuroinflammatory effect of Sophoraflavanone G from Sophora alopecuroides in LPS-activated BV2 microglia by MAPK, JAK/STAT and Nrf2/HO-1 signaling pathways.Phytomedicine. 2016 Dec 1;23(13):1629-1637. doi: 10.1016/j.phymed.2016.10.007. Epub 2016 Oct 14. Phytomedicine. 2016. PMID: 27823627
-
Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases.Molecules. 2022 May 17;27(10):3194. doi: 10.3390/molecules27103194. Molecules. 2022. PMID: 35630670 Free PMC article. Review.
-
Is cyclooxygenase-1 involved in neuroinflammation?J Neurosci Res. 2021 Nov;99(11):2976-2998. doi: 10.1002/jnr.24934. Epub 2021 Aug 4. J Neurosci Res. 2021. PMID: 34346520 Free PMC article. Review.
Cited by
-
Chemical Composition and Potential Properties in Mental Illness (Anxiety, Depression and Insomnia) of Agarwood Essential Oil: A Review.Molecules. 2022 Jul 15;27(14):4528. doi: 10.3390/molecules27144528. Molecules. 2022. PMID: 35889407 Free PMC article. Review.
-
Central role of microglia in sepsis-associated encephalopathy: From mechanism to therapy.Front Immunol. 2022 Jul 26;13:929316. doi: 10.3389/fimmu.2022.929316. eCollection 2022. Front Immunol. 2022. PMID: 35958583 Free PMC article. Review.
-
Characterization of IMG Microglial Cell Line as a Valuable In Vitro Tool for NLRP3 Inflammasome Studies.Cell Mol Neurobiol. 2023 Jul;43(5):2053-2069. doi: 10.1007/s10571-022-01285-6. Epub 2022 Sep 26. Cell Mol Neurobiol. 2023. PMID: 36163404 Free PMC article.
-
Plant-Based Bioactive Molecules in Improving Health and Preventing Lifestyle Diseases.Int J Mol Sci. 2021 Mar 15;22(6):2991. doi: 10.3390/ijms22062991. Int J Mol Sci. 2021. PMID: 33804225 Free PMC article.
-
Aging-induced dysbiosis worsens sepsis severity but is attenuated by probiotics in D-galactose-administered mice with cecal ligation and puncture model.PLoS One. 2024 Oct 18;19(10):e0311774. doi: 10.1371/journal.pone.0311774. eCollection 2024. PLoS One. 2024. PMID: 39423218 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

