Instrumentation-Free Semiquantitative Immunoanalysis Using a Specially Patterned Lateral Flow Assay Device
- PMID: 32751808
- PMCID: PMC7460358
- DOI: 10.3390/bios10080087
Instrumentation-Free Semiquantitative Immunoanalysis Using a Specially Patterned Lateral Flow Assay Device
Abstract
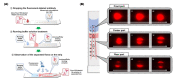
In traditional colorimetric lateral flow immunoassay (LFI) using gold nanoparticles (AuNPs) as a probe, additional optical transducers are required to quantify the signal intensity of the test line because it presents as a single red-colored line. In order to eliminate external equipment, the LFI signal should be quantifiable by the naked eye without the involvement of optical instruments. Given this objective, the single line test zone of conventional LFI was converted to several spots that formed herringbone patterns. When the sandwich immunoassay was performed on a newly developed semi-quantitative (SQ)-LFI system using AuNPs as an optical probe, the spots were colorized and the number of colored spots increased proportionally with the analyte concentration. By counting the number of colored spots, the analyte concentration can be easily estimated with the naked eye. To demonstrate the applicability of the SQ-LFI system in practical immunoanalysis, microalbumin, which is a diagnostic marker for renal failure, was analyzed using microalbumin-spiked artificial urine samples. Using the SQ-LFI system, the calibration results for artificial urine-based microalbumin were studied, ranging from 0 to 500 μg/mL, covering the required clinical detection range, and the limit of detection (LOD) value was calculated to be 15.5 μg/mL. Thus, the SQ-LFI system provides an avenue for the realization of an efficient quantification diagnostic device in resource-limited conditions.
Keywords: instrument-free quantitative analysis; lateral flow immunoassay; microalbuminuria; renal failure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Carbon nanotube-based lateral flow immunoassay for ultrasensitive detection of proteins: application to the determination of IgG.Mikrochim Acta. 2019 Jun 13;186(7):436. doi: 10.1007/s00604-019-3508-4. Mikrochim Acta. 2019. PMID: 31197469
-
Highly sensitive protein detection using recombinant spores and lateral flow immunoassay.Anal Bioanal Chem. 2021 Mar;413(8):2235-2246. doi: 10.1007/s00216-021-03195-w. Epub 2021 Feb 19. Anal Bioanal Chem. 2021. PMID: 33608751
-
Electrochemiluminescence-Incorporated Lateral Flow Immunosensors Using Ru(bpy)32+-Labeled Gold Nanoparticles for the Full-Range Detection of Physiological C-Reactive Protein Levels.Anal Chem. 2021 Jun 8;93(22):7925-7932. doi: 10.1021/acs.analchem.1c00623. Epub 2021 May 25. Anal Chem. 2021. PMID: 34032406
-
Utility of a Lateral Flow Immunoassay (LFI) to Detect Burkholderia pseudomallei in Soil Samples.PLoS Negl Trop Dis. 2016 Dec 14;10(12):e0005204. doi: 10.1371/journal.pntd.0005204. eCollection 2016 Dec. PLoS Negl Trop Dis. 2016. PMID: 27973567 Free PMC article.
-
Smartphone based lateral flow immunoassay quantifications.J Immunol Methods. 2024 Oct;533:113745. doi: 10.1016/j.jim.2024.113745. Epub 2024 Aug 22. J Immunol Methods. 2024. PMID: 39173705 Review.
Cited by
-
Progress in Biosensors for the Point-of-Care Diagnosis of COVID-19.Sensors (Basel). 2022 Sep 29;22(19):7423. doi: 10.3390/s22197423. Sensors (Basel). 2022. PMID: 36236521 Free PMC article. Review.
-
Recent Developments in Paper-Based Sensors with Instrument-Free Signal Readout Technologies (2020-2023).Biosensors (Basel). 2024 Jan 11;14(1):36. doi: 10.3390/bios14010036. Biosensors (Basel). 2024. PMID: 38248413 Free PMC article. Review.
-
ENaC Biomarker Detection in Platelets Using a Lateral Flow Immunoassay: A Clinical Validation Study.Biosensors (Basel). 2025 Jun 20;15(7):399. doi: 10.3390/bios15070399. Biosensors (Basel). 2025. PMID: 40710049 Free PMC article.
-
Blind Spot: A Braille Patterned Novel Multiplex Lateral Flow Immunoassay Sensor Array for the Detection of Biothreat Agents.ACS Omega. 2021 Aug 23;6(35):22700-22708. doi: 10.1021/acsomega.1c02938. eCollection 2021 Sep 7. ACS Omega. 2021. PMID: 34514241 Free PMC article.
References
-
- Dhiman A., Kalra P., Bansal V., Bruno J.G., Sharma T.K. Aptamer-based point-of-care diagnostic platforms. Sens. Actuators B Chem. 2017;246:535–553. doi: 10.1016/j.snb.2017.02.060. - DOI
-
- Darwish N.T., Sekaran S.D., Khor S.M. Point-of-care tests: A review of advances in the emerging diagnostic tools for dengue virus infection. Sens. Actuators B Chem. 2018;255:3316–3331. doi: 10.1016/j.snb.2017.09.159. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

