Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. albicans and C. glabrata
- PMID: 32751915
- PMCID: PMC7559477
- DOI: 10.3390/jof6030124
Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. albicans and C. glabrata
Abstract
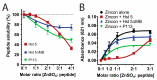
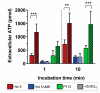
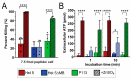
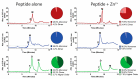
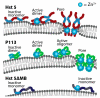
Histatin 5 (Hst 5) is an antimicrobial peptide produced in human saliva with antifungal activity for opportunistic pathogen Candida albicans. Hst 5 binds to multiple cations including dimerization-inducing zinc (Zn2+), although the function of this capability is incompletely understood. Hst 5 is taken up by C. albicans and acts on intracellular targets under metal-free conditions; however, Zn2+ is abundant in saliva and may functionally affect Hst 5. We hypothesized that Zn2+ binding would induce membrane-disrupting pores through dimerization. Through the use of Hst 5 and two derivatives, P113 (AA 4-15 of Hst 5) and Hst 5ΔMB (AA 1-3 and 15-19 mutated to Glu), we determined that Zn2+ significantly increases killing activity of Hst 5 and P113 for both C. albicans and Candida glabrata. Cell association assays determined that Zn2+ did not impact initial surface binding by the peptides, but Zn2+ did decrease cell association due to active peptide uptake. ATP efflux assays with Zn2+ suggested rapid membrane permeabilization by Hst 5 and P113 and that Zn2+ affinity correlates to higher membrane disruption ability. High-performance liquid chromatography (HPLC) showed that the higher relative Zn2+ affinity of Hst 5 likely promotes dimerization. Together, these results suggest peptide assembly into fungicidal pore structures in the presence of Zn2+, representing a novel mechanism of action that has exciting potential to expand the list of Hst 5-susceptible pathogens.
Keywords: Candida albicans; Candida glabrata; Histatin 5; P113; membrane disruption; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Oppenheim F.G., Xu T., Mcmillian F.M., Levitz S.M., Diamond R.D., Offner G.D., Troxler R.F. Histatins, A novel family of histidine-rich proteins in human parotid secretion. J. Biol. Chem. 1988;263:7472–7477. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

