Synthesis and Biological Activity of Benzamides Substituted with Pyridine-Linked 1,2,4-Oxadiazole
- PMID: 32752024
- PMCID: PMC7435590
- DOI: 10.3390/molecules25153500
Synthesis and Biological Activity of Benzamides Substituted with Pyridine-Linked 1,2,4-Oxadiazole
Abstract
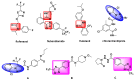
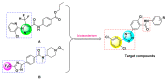
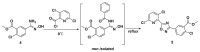
To find pesticidal lead compounds with high activity, a series of novel benzamides substituted with pyridine-linked 1,2,4-oxadiazole were designed by bioisosterism, and synthesized easily via esterification, cyanation, cyclization and aminolysis reactions. The structures of the target compounds were confirmed by 1H-NMR, 13C-NMR and HRMS. The preliminary bioassay showed that most compounds had good larvicidal activities against mosquito larvae at 10 mg/L, especially compound 7a, with a larvicidal activity as high as 100%, and even at 1 mg/L was still 40%; at 50 mg/L, all the target compounds showed good fungicidal activities against the eight tested fungi. Moreover, compound 7h exhibited better inhibitory activity (90.5%) than fluxapyroxad (63.6%) against Botrytis cinereal. Therefore, this type of compound can be further studied.
Keywords: 1,2,4-oxadiazole; amide compound; fungicidal activity; insecticidal activity; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Wang L., Dai F.Y., Zhu J., Dong K.K., Wang Y.L., Chen T. Synthesis and antibacterial activities of pleuromutilin derivatives with thiazole-5-carboxamide and thioether moiety. J. Chem. Res. 2011;35:313–316. doi: 10.3184/174751911X13057375208346. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources