How to RESPOND to Modern Challenges for People Living with HIV: A Profile for a New Cohort Consortium
- PMID: 32752044
- PMCID: PMC7464889
- DOI: 10.3390/microorganisms8081164
How to RESPOND to Modern Challenges for People Living with HIV: A Profile for a New Cohort Consortium
Abstract
Background: the International Cohort Consortium of Infectious Disease (RESPOND) is a collaboration dedicated to research on HIV and other infectious diseases.
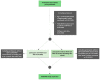
Methods: RESPOND is a flexible organization, with several independent substudies operating under one shared governance. HIV-related variables, including full antiretroviral therapy (ART) history, are collected annually for all participants and merged with substudy specific data into a shared data pool. Incident clinical events are reported using standardized forms. Prospective follow-up started 1/10/17 (enrolment) with retrospective data collected back to 01/01/12.
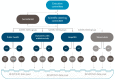
Results: Overall, 17 cohorts from Europe and Australia provided data on 26,258 people living with HIV (PLWH). The majority (43.3%) of the population were white, with men-sex-with-men accounting for 43.3% of the risk for HIV acquisition. The median age was 48 years (IQR 40-56) and 5.2% and 25.5% were known to be co-infected with hepatitis B or C. While 5.3% were ART-naïve, the median duration on ART was 10.1 years (4.8-17.6), with 89.5% having a VL <200 copies/mL and the median CD4 count being 621 cells/µL (438-830). Malignancies (n = 361) and cardiovascular disease (n = 168) were the predominant reported clinical events.
Conclusion: RESPOND's large, diverse study population and standardized clinical endpoints puts the consortium in a unique position to respond to the diverse modern challenges for PLWH.
Keywords: HIV; cohort; hepatitis; observational study; pharmacovigilance; public health; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Friis-Moller N., Weber R., Reiss P., Thiebaut R., Kirk O., d’Arminio Monforte A., Pradier C., Morfeldt L., Mateu S., Law M. Cardiovascular disease risk factors in HIV patients—Association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–1193. doi: 10.1097/00002030-200305230-00010. - DOI - PubMed
-
- Chêne G., Phillips A., Costagliola D., Sterne J.A., Furrer H., Del Amo J., Mocroft A., d’Arminio Monforte A., Dabis F., Miro J.M., et al. Cohort profile: Collaboration of observational HIV epidemiological research europe (COHERE) in EuroCoord. Int. J. Epidemiol. 2017;46:797–797n. doi: 10.1093/ije/dyw211. - DOI - PMC - PubMed
-
- RESPOND Consortium Description v1.0 2019. [(accessed on 27 July 2020)]; Available online: https://chip.dk/Portals/0/files/RESPOND/RESPOND_Consortium-description_V....
LinkOut - more resources
Full Text Sources
Research Materials

