Xeno-Free In Vitro Cultivation and Osteogenic Differentiation of hAD-MSCs on Resorbable 3D Printed RESOMER®
- PMID: 32752065
- PMCID: PMC7436127
- DOI: 10.3390/ma13153399
Xeno-Free In Vitro Cultivation and Osteogenic Differentiation of hAD-MSCs on Resorbable 3D Printed RESOMER®
Abstract
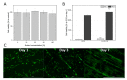
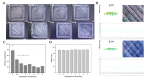
The development of alloplastic resorbable materials can revolutionize the field of implantation technology in regenerative medicine. Additional opportunities to colonize the three-dimensionally (3D) printed constructs with the patient's own cells prior to implantation can improve the regeneration process but requires optimization of cultivation protocols. Human platelet lysate (hPL) has already proven to be a suitable replacement for fetal calf serum (FCS) in 2D and 3D cell cultures. In this study, we investigated the in vitro biocompatibility of the printed RESOMER® Filament LG D1.75 materials as well as the osteogenic differentiation of human mesenchymal stem cells (hMSCs) cultivated on 3D printed constructs under the influence of different medium supplements (FCS, human serum (HS) and hPL). Additionally, the in vitro degradation of the material was studied over six months. We demonstrated that LG D1.75 is biocompatible and has no in vitro cytotoxic effects on hMSCs. Furthermore, hMSCs grown on the constructs could be differentiated into osteoblasts, especially supported by supplementation with hPL. Over six months under physiological in vitro conditions, a distinct degradation was observed, which, however, had no influence on the biocompatibility of the material. Thus, the overall suitability of the material LG D1.75 to produce 3D printed, resorbable bone implants and the promising use of hPL in the xeno-free cultivation of human MSCs on such implants for autologous transplantation have been demonstrated.
Keywords: 3D printing; RESOMER®; adipose tissue-derived mesenchymal stem cells (hAD-MSCs); fetal calve serum; human platelet lysate; human serum; in vitro biocompatibility; in vitro degradation; osteogenic differentiation; resorbable polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hull C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. No. 638905. U.S. Patent. 1984 Aug 8;
-
- Keyhan S.O., Fallahi H., Jahangirnia A., Amirzade-Iranaq M.T., Amirzade-Iranaq M.H. Application of 3-D Printing for Tissue Regeneration in Oral and Maxillofacial Surgery: What is Upcoming. In: Dobrzański L.A., editor. Biomaterials in Regenerative Medicine. InTech; London, UK: 2018.
LinkOut - more resources
Full Text Sources
Research Materials

