In Silico Study Identified Methotrexate Analog as Potential Inhibitor of Drug Resistant Human Dihydrofolate Reductase for Cancer Therapeutics
- PMID: 32752079
- PMCID: PMC7435474
- DOI: 10.3390/molecules25153510
In Silico Study Identified Methotrexate Analog as Potential Inhibitor of Drug Resistant Human Dihydrofolate Reductase for Cancer Therapeutics
Abstract
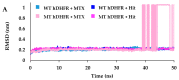
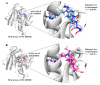
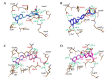
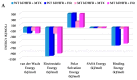
Drug resistance is a core issue in cancer chemotherapy. A known folate antagonist, methotrexate (MTX) inhibits human dihydrofolate reductase (hDHFR), the enzyme responsible for the catalysis of 7,8-dihydrofolate reduction to 5,6,7,8-tetrahydrofolate, in biosynthesis and cell proliferation. Structural change in the DHFR enzyme is a significant cause of resistance and the subsequent loss of MTX. In the current study, wild type hDHFR and double mutant (engineered variant) F31R/Q35E (PDB ID: 3EIG) were subject to computational study. Structure-based pharmacophore modeling was carried out for wild type (WT) and mutant (MT) (variant F31R/Q35E) hDHFR structures by generating ten models for each. Two pharmacophore models, WT-pharma and MT-pharma, were selected for further computations, and showed excellent ROC curve quality. Additionally, the selected pharmacophore models were validated by the Guner-Henry decoy test method, which yielded high goodness of fit for WT-hDHFR and MT-hDHFR. Using a SMILES string of MTX in ZINC15 with the selections of 'clean', in vitro and in vivo options, 32 MTX-analogs were obtained. Eight analogs were filtered out due to their drug-like properties by applying absorption, distribution, metabolism, excretion, and toxicity (ADMET) assessment tests and Lipinski's Rule of five. WT-pharma and MT-pharma were further employed as a 3D query in virtual screening with drug-like MTX analogs. Subsequently, seven screening hits along with a reference compound (MTX) were subjected to molecular docking in the active site of WT- and MT-hDHFR. Through a clustering analysis and examination of protein-ligand interactions, one compound was found with a ChemPLP fitness score greater than that of MTX (reference compound). Finally, a simulation of molecular dynamics (MD) identified an MTX analog which exhibited strong affinity for WT- and MT-hDHFR, with stable RMSD, hydrogen bonds (H-bonds) in the binding site and the lowest MM/PBSA binding free energy. In conclusion, we report on an MTX analog which is capable of inhibiting hDHFR in wild type form, as well as in cases where the enzyme acquires resistance to drugs during chemotherapy treatment.
Keywords: Drug resistance; human dihydrofolate reductase; methotrexate; molecular docking; molecular dynamics simulation.; pharmacophore modeling; virtual screening.
Conflict of interest statement
The authors declare no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





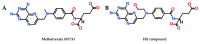
References
-
- Tran P.N., Tate C.J., Ridgway M.C., Saliba K.J., Kirk K., Maier A.G. Human dihydrofolate reductase influences the sensitivity of the malaria parasite Plasmodium falciparum to ketotifen - A cautionary tale in screening transgenic parasites. Int. J. Parasitol. Drugs Drug Resist. 2016;6:179–183. doi: 10.1016/j.ijpddr.2016.09.003. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

