Pyrazoline Hybrids as Promising Anticancer Agents: An Up-to-Date Overview
- PMID: 32752126
- PMCID: PMC7432644
- DOI: 10.3390/ijms21155507
Pyrazoline Hybrids as Promising Anticancer Agents: An Up-to-Date Overview
Abstract
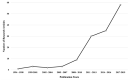
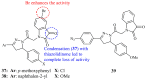
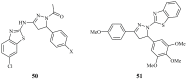
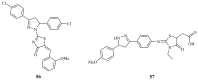
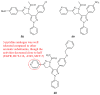
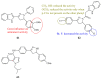
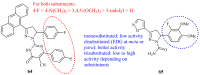
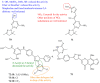
Pyrazolines are five-membered heterocycles possessing two adjacent nitrogens. They have attracted significant attention from organic and medicinal chemists due to their potent biological activities and the numerous possibilities for structural diversification. In the last decade, they have been intensively studied as targets for potential anticancer therapeutics, producing a steady yearly rise in the number of published research articles. Many pyrazoline derivatives have shown remarkable cytotoxic activities in the form of heterocyclic or non-heterocyclic based hybrids, such as with coumarins, triazoles, and steroids. The enormous amount of related literature in the last 5 years prompted us to collect all these published data from screening against cancer cell lines, or protein targets like EGFR and structure activity relationship studies. Therefore, in the present review, a comprehensive account of the compounds containing the pyrazoline nucleus will be provided. The chemical groups and the structural modifications responsible for the activity will be highlighted. Moreover, emphasis will be given on recent examples from the literature and on the work of research groups that have played a key role in the development of this field.
Keywords: anticancer; antitumor; conjugates; cytotoxicity; hybrid compounds; nitrogen heterocycles; pyrazolines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



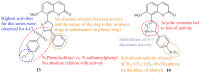

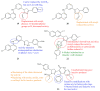
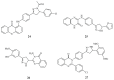

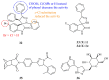





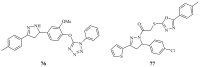


References
-
- World Health Organization Cancer: Key Facts. [(accessed on 15 March 2020)]; Available online: http://www.who.int/news-room/fact-sheets/detail/cancer.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

