Antibody-Drug Conjugates: The New Frontier of Chemotherapy
- PMID: 32752132
- PMCID: PMC7432430
- DOI: 10.3390/ijms21155510
Antibody-Drug Conjugates: The New Frontier of Chemotherapy
Abstract
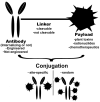
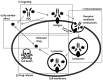
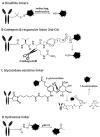
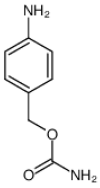
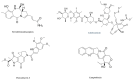
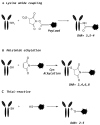
In recent years, antibody-drug conjugates (ADCs) have become promising antitumor agents to be used as one of the tools in personalized cancer medicine. ADCs are comprised of a drug with cytotoxic activity cross-linked to a monoclonal antibody, targeting antigens expressed at higher levels on tumor cells than on normal cells. By providing a selective targeting mechanism for cytotoxic drugs, ADCs improve the therapeutic index in clinical practice. In this review, the chemistry of ADC linker conjugation together with strategies adopted to improve antibody tolerability (by reducing antigenicity) are examined, with particular attention to ADCs approved by the regulatory agencies (the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA)) for treating cancer patients. Recent developments in engineering Immunoglobulin (Ig) genes and antibody humanization have greatly reduced some of the problems of the first generation of ADCs, beset by problems, such as random coupling of the payload and immunogenicity of the antibody. ADC development and clinical use is a fast, evolving area, and will likely prove an important modality for the treatment of cancer in the near future.
Keywords: Antibody-Drug Conjugate; Mabs; cancer therapy; cross-linking; drug targeting; payload.
Conflict of interest statement
G.S. and S.I. are shareholders of Mediapharma s.r.l. The other authors have no potential conflict of interest to report.
Figures
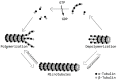
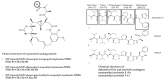
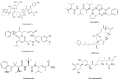
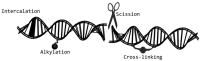
References
-
- Hoffmann R.M., Coumbe B.G.T., Josephs D.H., Mele S., Ilieva K.M., Cheung A., Tutt A.N., Spicer J.F., Thurston D.E., Crescioli S., et al. Antibody structure and engineering considerations for the design and function of Antibody Drug Conjugates (ADCs) OncoImmunology. 2018;7:e1395127. doi: 10.1080/2162402X.2017.1395127. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

