In Vivo Anti-inflammatory Potential of Viscozyme®-Treated Jujube Fruit
- PMID: 32752184
- PMCID: PMC7466189
- DOI: 10.3390/foods9081033
In Vivo Anti-inflammatory Potential of Viscozyme®-Treated Jujube Fruit
Abstract
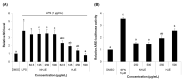
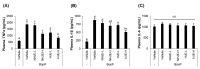
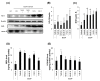
The fruit of Ziziphus jujuba, commonly called jujube, has long been consumed for its health benefits. The aim of this study was to examine the protective effect of dietary supplementation of enzymatically hydrolyzed jujube against lung inflammation in mice. The macerated flesh of jujube was extracted with aqueous ethanol before and after Viscozyme treatment. The extract of enzyme-treated jujube, called herein hydrolyzed jujube extract (HJE), contained higher levels of quercetin, total phenolics, and flavonoids, and exhibited more effective radical-scavenging abilities in comparison to non-hydrolyzed jujube extract (NHJE). HJE treatment decreased production of inflammation-associated molecules, including nitric oxide and pro-inflammatory cytokines from activated Raw 264.7 or differentiated THP-1 cells. HJE treatment also reduced expression of nuclear factor-κB and its downstream proteins in A549 human lung epithelial cells. Moreover, oral supplementation of 1.5 g of HJE per kg of body weight (BW) attenuated histological lung damage, decreased plasma cytokines, and inhibited expression of inflammatory proteins and oxidative stress mediators in the lungs of mice exposed to benzo(a)pyrene at 50 mg/kg BW. Expression levels of antioxidant and cytoprotective factors, such as nuclear factor erythroid-derived 2-related factor 2 and heme oxygenase-1, were increased in lung and liver tissues from mice treated with HJE, compared to mice fed NHJE. These findings indicate that dietary HJE can reduce benzo(a)pyrene-induced lung inflammation by inhibiting cytokine release from macrophages and promoting antioxidant defenses in vivo.
Keywords: HO-1; Jujube; NF-κB; Nrf2; anti-inflammation; hydrolysis; lung.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures




References
-
- Vlahos R., Bozinovski S., Jones J., Powell J., Gras J., Lilja A., Hansen M.J., Gualano R.C., Irving L., Anderson G. Differential protease, innate immunity, and NF-κB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L931–L945. doi: 10.1152/ajplung.00201.2005. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

