Expression of a Shiga-Like Toxin during Plastic Colonization by Two Multidrug-Resistant Bacteria, Aeromonas hydrophila RIT668 and Citrobacter freundii RIT669, Isolated from Endangered Turtles (Clemmys guttata)
- PMID: 32752245
- PMCID: PMC7465454
- DOI: 10.3390/microorganisms8081172
Expression of a Shiga-Like Toxin during Plastic Colonization by Two Multidrug-Resistant Bacteria, Aeromonas hydrophila RIT668 and Citrobacter freundii RIT669, Isolated from Endangered Turtles (Clemmys guttata)
Abstract
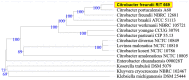
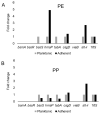
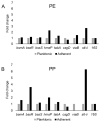
Aeromonas hydrophila RIT668 and Citrobacter freundii RIT669 were isolated from endangered spotted turtles (Clemmys guttata). Whole-genome sequencing, annotation and phylogenetic analyses of the genomes revealed that the closest relative of RIT668 is A. hydrophila ATCC 7966 and Citrobacter portucalensis A60 for RIT669. Resistome analysis showed that A. hydrophila and C. freundii harbor six and 19 different antibiotic resistance genes, respectively. Both bacteria colonize polyethylene and polypropylene, which are common plastics, found in the environment and are used to fabricate medical devices. The expression of six biofilm-related genes-biofilm peroxide resistance protein (bsmA), biofilm formation regulatory protein subunit R (bssR), biofilm formation regulatory protein subunit S (bssS), biofilm formation regulator (hmsP), toxin-antitoxin biofilm protein (tabA) and transcriptional activator of curli operon (csgD)-and two virulence factors-Vi antigen-related gene (viaB) and Shiga-like toxin (slt-II)-was investigated by RT-PCR. A. hydrophila displayed a >2-fold increase in slt-II expression in cells adhering to both polymers, C. freundii adhering on polyethylene displayed a >2-fold, and on polypropylene a >6-fold upregulation of slt-II. Thus, the two new isolates are potential pathogens owing to their drug resistance, surface colonization and upregulation of a slt-II-type diarrheal toxin on polymer surfaces.
Keywords: Aeromonas; Citrobacter; Shiga-like toxin; antibiotic resistance; biofilm; plastic; turtle; whole-genome sequencing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

