Iron Metabolism in Obesity and Metabolic Syndrome
- PMID: 32752277
- PMCID: PMC7432525
- DOI: 10.3390/ijms21155529
Iron Metabolism in Obesity and Metabolic Syndrome
Abstract
Obesity is an excessive adipose tissue accumulation that may have detrimental effects on health. Particularly, childhood obesity has become one of the main public health problems in the 21st century, since its prevalence has widely increased in recent years. Childhood obesity is intimately related to the development of several comorbidities such as nonalcoholic fatty liver disease, dyslipidemia, type 2 diabetes mellitus, non-congenital cardiovascular disease, chronic inflammation and anemia, among others. Within this tangled interplay between these comorbidities and associated pathological conditions, obesity has been closely linked to important perturbations in iron metabolism. Iron is the second most abundant metal on Earth, but its bioavailability is hampered by its ability to form highly insoluble oxides, with iron deficiency being the most common nutritional disorder. Although every living organism requires iron, it may also cause toxic oxygen damage by generating oxygen free radicals through the Fenton reaction. Thus, iron homeostasis and metabolism must be tightly regulated in humans at every level (i.e., absorption, storage, transport, recycling). Dysregulation of any step involved in iron metabolism may lead to iron deficiencies and, eventually, to the anemic state related to obesity. In this review article, we summarize the existent evidence on the role of the most recently described components of iron metabolism and their alterations in obesity.
Keywords: anemia; childhood obesity; iron; metabolic syndrome; metabolism; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
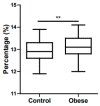
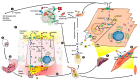
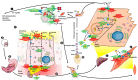
References
-
- World Health Organization—Obesity. [(accessed on 29 June 2020)]; Available online: https://www.who.int/health-topics/obesity#tab=tab_1.
-
- World Health Organization—Childhood Obesity. [(accessed on 29 June 2020)]; Available online: https://www.who.int/dietphysicalactivity/childhood/en/
-
- Martos-Moreno G.Á., Gil-Campos M., Bueno G., Bahillo P., Bernal S., Feliu A., Lechuga-Sancho A.M., Palomo E., Ruiz R., Vela A. Las alteraciones metabólicas asociadas a la obesidad están ya presentes en los primeros años de vida: Estudio colaborativo español. Nutr. Hosp. 2014;30:787–793. doi: 10.3305/nh.2014.30.4.7661. - DOI - PubMed

