Cisplatin Decreases ENaC Activity Contributing to Renal Salt Wasting Syndrome
- PMID: 32752278
- PMCID: PMC7464492
- DOI: 10.3390/cancers12082140
Cisplatin Decreases ENaC Activity Contributing to Renal Salt Wasting Syndrome
Abstract
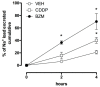
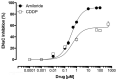
Cisplatin (CDDP) is an important anticancer drug. A common side effect of CDDP is renal salt and water-wasting syndrome (RSWS). The origin of RSWS is obscure. Emerging evidence, though, suggests that broad inhibition of sodium transport proteins by CDDP may result in decreases in tubular reabsorption, causing increases in sodium and water excretion. In this sense, CDDP would be acting like a diuretic. The effect of CDDP on the epithelial Na+ channel (ENaC), which is the final arbiter fine-tuning renal Na+ excretion, is unknown. We test here whether CDDP affects ENaC to promote renal salt and water excretion. The effects of CDDP and benzamil (BZM), a blocker of ENaC, on excretion of a sodium load were quantified. Similar to BZM, CDDP facilitated renal Na+ excretion. To directly quantify the effects on ENaC, principal cells in split-open tubules were patch clamped. CDDP, at doses comparable to those used for chemotherapy (1.5 µM), significantly decreased ENaC activity in native tubules. To further elaborate on this mechanism, the dose-dependent effects of CDDP on mouse ENaC (mENaC) heterologously expressed in Chinese Hamster Ovary (CHO) cells were tested using patch clamping. As in native tubules, CDDP significantly decreased the activity of mENaC expressed in CHO cells. Dose-response curves and competition with amiloride identified CDDP as a weak inhibitor of ENaC (apparent IC50 = 1 µM) that competes with amiloride for inhibition of the channel, weakening the inhibitory actions of the latter. Such observations are consistent with CDDP being a partial modulator of ENaC, which possibly has a binding site that overlaps with that of amiloride. These findings are consistent with inhibition of ENaC by CDDP contributing to the RSWS caused by this important chemotherapy drug.
Keywords: Deg/ENaC channels; chemotherapy; diuretic; hypertension; pseduohypoaldosteronism; renal sodium excretion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Chu G. Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. J. Biol. Chem. 1994;269:787–790. - PubMed
-
- Ciarimboli G., Ludwig T., Lang D., Pavenstädt H., Koepsell H., Piechota H.-J., Haier J., Jaehde U., Zisowsky J., Schlatter E. Cisplatin Nephrotoxicity Is Critically Mediated via the Human Organic Cation Transporter 2. Am. J. Pathol. 2005;167:1477–1484. doi: 10.1016/S0002-9440(10)61234-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Research Materials
