Current status and strategic possibilities on potential use of combinational drug therapy against COVID-19 caused by SARS-CoV-2
- PMID: 32752944
- PMCID: PMC7484586
- DOI: 10.1080/07391102.2020.1802345
Current status and strategic possibilities on potential use of combinational drug therapy against COVID-19 caused by SARS-CoV-2
Abstract
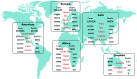
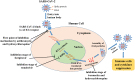
The spread of new coronavirus infection starting December 2019 as novel SARS-CoV-2, identified as the causing agent of COVID-19, has affected all over the world and been declared as pandemic. Approximately, more than 8,807,398 confirmed cases of COVID-19 infection and 464,483 deaths have been reported globally till the end of 21 June 2020. Until now, there is no specific drug therapy or vaccine available for the treatment of COVID-19. However, some potential antimalarial drugs like hydroxychloroquine and azithromycin, antifilarial drug ivermectin and antiviral drugs have been tested by many research groups worldwide for their possible effect against the COVID-19. Hydroxychloroquine and ivermectin have been identified to act by creating the acidic condition in cells and inhibiting the importin (IMPα/β1) mediated viral import. There is a possibility that some other antimalarial drugs/antibiotics in combination with immunomodulators may help in combatting this pandemic disease. Therefore, this review focuses on the current use of various drugs as single agents (hydroxychloroquine, ivermectin, azithromycin, favipiravir, remdesivir, umifenovir, teicoplanin, nitazoxanide, doxycycline, and dexamethasone) or in combinations with immunomodulators additionally. Furthermore, possible mode of action, efficacy and current stage of clinical trials of various drug combinations against COVID-19 disease has also been discussed in detail.Communicated by Ramaswamy H. Sarma.
Keywords: ACE2 receptor; COVID-19; Coronavirus; SARS-CoV-2; immunomodulators.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures

References
-
- Aanouz, I., Belhassan, A., El-Khatabi, K., Lakhlifi, T., El-Ldrissi, M., & Bouachrine, M. (2020). Moroccan Medicinal plants as inhibitors against SARS-CoV-2 main protease: Computational investigations. Journal of Biomolecular Structure and Dynamics, 6, 1–9. 10.1080/07391102.2020.1758790 - DOI - PMC - PubMed
-
- Acter, T., Uddin, N., Das, J., Akhter, A., Choudhury, T. R., & Kim, S. (2020). Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. The Science of the Total Environment, 730, 138996. 10.1016/j.scitotenv.2020.138996 - DOI - PMC - PubMed
-
- Adeoye, A. O., Oso, B. J., Olaoye, I. F., Tijjani, H., & Adebayo, A. I. (2020). Repurposing of chloroquine and some clinically approved antiviral drugs as effective therapeutics to prevent cellular entry and replication of coronavirus. Journal of Biomolecular Structure and Dynamics, 15, 1–11. 10.1080/07391102.2020.1765876 - DOI - PMC - PubMed
-
- Agostini, M. L., Andres, E. L., Sims, A. C., Graham, R. L., Sheahan, T. P., Lu, X., Smith, E. C., Case, J. B., Feng, J. Y., Jordan, R., Ray, A. S., Cihlar, T., Siegel, D., Mackman, R. L., Clarke, M. O., Baric, R. S., & Denison, M. R. (2018). Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 9, 9(2), e00221. 10.1128/mBio.00221-18 - DOI - PMC - PubMed
-
- Al-Kofahi, M., Jacobson, P., Boulware, D. R., Matas, A., Kandaswamy, R., Jaber, M. M., Rajasingham, R., Young, J. H., & Nicol, M. R. (2020). Finding the dose for hydroxychloroquine prophylaxis for COVID-19; the desperate search for effectiveness. Clinical Pharmacology & Therapeutics, 1–4. 10.1002/cpt.1874 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
