The Tomato Guanylate-Binding Protein SlGBP1 Enables Fruit Tissue Differentiation by Maintaining Endopolyploid Cells in a Non-Proliferative State
- PMID: 32753430
- PMCID: PMC7534463
- DOI: 10.1105/tpc.20.00245
The Tomato Guanylate-Binding Protein SlGBP1 Enables Fruit Tissue Differentiation by Maintaining Endopolyploid Cells in a Non-Proliferative State
Abstract
Cell fate maintenance is an integral part of plant cell differentiation and the production of functional cells, tissues, and organs. Fleshy fruit development is characterized by the accumulation of water and solutes in the enlarging cells of parenchymatous tissues. In tomato (Solanum lycopersicum), this process is associated with endoreduplication in mesocarp cells. The mechanisms that preserve this developmental program, once initiated, remain unknown. We show here that analysis of a previously identified tomato ethyl methanesulfonate-induced mutant that exhibits abnormal mesocarp cell differentiation could help elucidate determinants of fruit cell fate maintenance. We identified and validated the causal locus through mapping-by-sequencing and gene editing, respectively, and performed metabolic, cellular, and transcriptomic analyses of the mutant phenotype. The data indicate that disruption of the SlGBP1 gene, encoding GUANYLATE BINDING PROTEIN1, induces early termination of endoreduplication followed by late divisions of polyploid mesocarp cells, which consequently acquire the characteristics of young proliferative cells. This study reveals a crucial role of plant GBPs in the control of cell cycle genes, and thus, in cell fate maintenance. We propose that SlGBP1 acts as an inhibitor of cell division, a function conserved with the human hGBP-1 protein.
© 2020 American Society of Plant Biologists. All rights reserved.
Figures
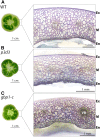
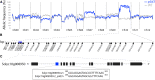
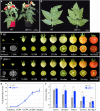





Comment in
-
Tempting Fate: A Guanylate-Binding Protein Maintains Tomato Fruit Cell Differentiation.Plant Cell. 2020 Oct;32(10):3047-3048. doi: 10.1105/tpc.20.00627. Epub 2020 Aug 13. Plant Cell. 2020. PMID: 32796122 Free PMC article. No abstract available.
References
-
- Akihiro, T. , et al. (2008). Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol. 49: 1378–1389. - PubMed
-
- Baloban, M. , Vanstraelen, M. , Tarayre, S. , Reuzeau, C. , Cultrone, A. , Mergaert, P. , Kondorosi, E. (2013). Complementary and dose-dependent action of AtCCS52A isoforms in endoreduplication and plant size control. New Phytol. 198: 1049–1059. - PubMed
-
- Benjamini, Y. , Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57: 289–300.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

