Sediment microbial fuel cells as a barrier to sulfide accumulation and their potential for sediment remediation beneath aquaculture pens
- PMID: 32753606
- PMCID: PMC7403589
- DOI: 10.1038/s41598-020-70002-4
Sediment microbial fuel cells as a barrier to sulfide accumulation and their potential for sediment remediation beneath aquaculture pens
Abstract
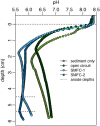
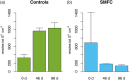
Sediment microbial fuel cells (SMFCs) generate electricity through the oxidation of reduced compounds, such as sulfide or organic carbon compounds, buried in anoxic sediments. The ability to remove sulfide suggests their use in the remediation of sediments impacted by point source organic matter loading, such as occurs beneath open pen aquaculture farms. However, for SMFCs to be a viable technology they must remove sulfide at a scale relevant to the environmental contamination and their impact on the sediment geochemistry as a whole must be evaluated. Here we address these issues through a laboratory microcosm experiment. Two SMFCs placed in high organic matter sediments were operated for 96 days and compared to open circuit and sediment only controls. The impact on sediment geochemistry was evaluated with microsensor profiling for oxygen, sulfide, and pH. The SMFCs had no discernable effect on oxygen profiles, however porewater sulfide was significantly lower in the sediment microcosms with functioning SMFCs than those without. Depth integrated sulfide inventories in the SMFCs were only 20% that of the controls. However, the SMFCs also lowered pH in the sediments and the consequences of this acidification on sediment geochemistry should be considered if developing SMFCs for remediation. The data presented here indicate that SMFCs have potential for the remediation of sulfidic sediments around aquaculture operations.
Conflict of interest statement
C. Algar and G. Wanger have filed a United States provisional patent (62/724861) related to this work. A. Howard and C. Ward declare no competing interests.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources

